


1. Sch 12041
1. Paxipam
2. Sch 12041
3. 23092-17-3
4. Halazepamum [inn-latin]
5. Schering 12041
6. Sch-12041
7. Halazepam Civ
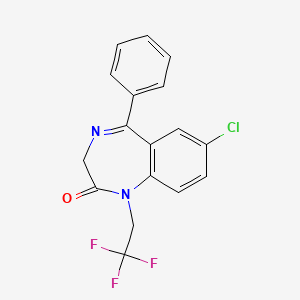
8. 7-chloro-1,3-dihydro-5-phenyl-1-(2,2,2-trifluoroethyl)-2h-1,4-benzodiazepin-2-one
9. 7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-3h-1,4-benzodiazepin-2-one
10. 2h-1,4-benzodiazepin-2-one, 7-chloro-1,3-dihydro-5-phenyl-1-(2,2,2-trifluoroethyl)-
11. Pacinone
12. 7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-1,3-dihydro-2h-1,4-benzodiazepin-2-one
13. 320yc168lf
14. Halazepamum
15. 2h-1,4-benzodiazepin-2-one, 1,3-dihydro-7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-
16. 7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-2,3-dihydro-1h-1,4-benzodiazepin-2-one
17. Paxipam (tn)
18. Halazepam (usan/inn)
19. Einecs 245-425-4
20. Brn 0898553
21. Alapryl
22. Halezepam
23. Unii-320yc168lf
24. Dea No. 2762
25. Halazepam [usan:usp:inn:ban]
26. Halazepam [inn]
27. Halazepam [mi]
28. Halazepam [usan]
29. Halazepam [vandf]
30. Chembl970
31. Halazepam [mart.]
32. Halazepam [who-dd]
33. Schembl78995
34. 5-24-04-00303 (beilstein Handbook Reference)
35. Chebi:5603
36. Gtpl7195
37. Halazepam [orange Book]
38. Dtxsid5023118
39. Zinc537811
40. Bdbm50408018
41. Db00801
42. D00338
43. Q5641126
44. 7-chloro-5-phenyl-1-(2,2,2-trifluorethyl)-1h-1,4-benzodiazepin-2(3h)-on
| Molecular Weight | 352.7 g/mol |
|---|---|
| Molecular Formula | C17H12ClF3N2O |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 352.0590252 g/mol |
| Monoisotopic Mass | 352.0590252 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 503 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to relieve anxiety, nervousness, and tension associated with anxiety disorders.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA13 - Halazepam
Hepatic.
Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.