


1. Alcinonide
2. Dermalog
3. Halciderm
4. Halcimat
5. Halog
6. Sq 18,566
7. Sq-18,566
8. Sq18,566
1. 3093-35-4
2. Halciderm
3. Halcimat
4. Halog
5. Halcinonida
6. Sq 18566
7. Si86v6qneg
8. Halcort
9. Sq-18566
10. Nsc-758413
11. So-18566
12. Dsstox_cid_25375
13. Dsstox_rid_80836
14. Dsstox_gsid_45375
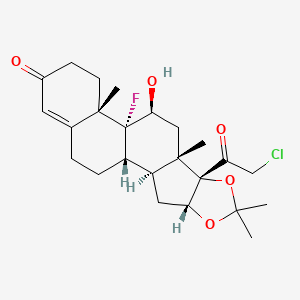
15. (1s,2s,4r,8s,9s,11s,12r,13s)-8-(2-chloroacetyl)-12-fluoro-11-hydroxy-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.02,9.04,8.013,18]icos-17-en-16-one
16. (4r,8s)-8-(2-chloroacetyl)-12-fluoro-11-hydroxy-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.02,9.04,8.013,18]icos-17-en-16-one
17. Sq-18,566
18. Betacorton
19. Halcinonidum
20. Halog-e
21. Ascochrom
22. Volog
23. (1s,2s,4r,8s,9s,11s,12r,13s)-8-(2-chloroacetyl)-12-fluoro-11-hydroxy-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icos-17-en-16-one
24. Unii-si86v6qneg
25. Halcinonidum [inn-latin]
26. Halcinonida [inn-spanish]
27. Ncgc00016621-01
28. (11?,16?)-21-chloro-9-fluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-pregn-4-ene-3,20-dione
29. Cas-3093-35-4
30. Einecs 221-439-6
31. Prestwick_1069
32. Halog (tn)
33. Brn 1358242
34. Halcinonide [usan:usp:inn:ban:jan]
35. Halcinonide [mi]
36. Prestwick0_000655
37. Prestwick1_000655
38. Prestwick2_000655
39. Prestwick3_000655
40. Halcinonide [inn]
41. Halcinonide [jan]
42. Halcinonide [usan]
43. Halcinonide [vandf]
44. Schembl4335
45. Halcinonide [mart.]
46. Bspbio_000689
47. Halcinonide [usp-rs]
48. Halcinonide [who-dd]
49. 5-19-06-00301 (beilstein Handbook Reference)
50. Mls002153935
51. Halcinonide (jan/usp/inn)
52. Spbio_002610
53. Bpbio1_000759
54. Chembl1200845
55. Dtxsid6045375
56. Chebi:31663
57. Halcinonide [orange Book]
58. Hms1570c11
59. Hms2097c11
60. Hms2230o07
61. Hms3714c11
62. Halcinonide [usp Monograph]
63. 21-chloro-9-fluoro-11.beta.,16.alpha.,17-trihydroxypregn-4-ene-3,20-dione Cyclic 16,17-acetal With Acetone
64. Hy-b0877
65. Pregn-4-ene-3,20-dione, 21-chloro-9-fluoro-11-hydroxy-16,17-((1-methylethylidene)bis(oxy))-, (11.beta.,16.alpha.)-
66. Zinc4213474
67. Tox21_110530
68. S4098
69. Akos015962797
70. Tox21_110530_1
71. Ac-1114
72. Ac-1774
73. Ccg-220655
74. Db06786
75. Nsc 758413
76. N6-(trifluoroacetyl)-l-lysyl-l-proline
77. Ncgc00179475-01
78. Ncgc00179475-03
79. 21-chloro-9-fluoro-11beta,16alpha,17-trihydroxypregn-4-ene-3,20-dione Cyclic 16,17-acetal With Acetone
80. As-74757
81. Pregn-4-ene-3,20-dione, 21-chloro-9-fluoro-11-hydroxy-16,17-((1-methylethylidene)bis(oxy))-, (11beta,16alpha)-
82. Smr001233277
83. So 18566
84. H1672
85. C74589
86. D01308
87. 093h354
88. Q425991
89. Q-201180
90. Brd-k81709173-001-03-8
91. Halcinonide, United States Pharmacopeia (usp) Reference Standard
92. (1s,2s,4r,8s,9s,11s,12r,13s)-8-(2-chloroacetyl)-12-fluoro-11-hydroxy-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0(2),?.0?,?.0(1)(3),(1)?]icos-17-en-16-one
93. (4as,4br,5s,6as,6bs,9ar,10as,10bs)-6b-(chloroacetyl)-4b-fluoro-5-hydroxy-4a,6a,8,8-tetramethyl-3,4,4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-tetradecahydro-2h-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one
94. Pregn-4-ene-3,20-dione, 21-chloro-9-fluoro-11-beta,16-alpha,17-trihydroxy-, Cyclic16,17-acetal With Acetone
95. Pregn-4-ene-3,20-dione,21-chloro-9-fluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(11b,16a)-
| Molecular Weight | 455.0 g/mol |
|---|---|
| Molecular Formula | C24H32ClFO5 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 454.1922300 g/mol |
| Monoisotopic Mass | 454.1922300 g/mol |
| Topological Polar Surface Area | 72.8 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 887 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Halog |
| PubMed Health | Halcinonide (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Strong |
| Drug Label | The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include halcinonide. Halcinonide is designated chemically as 21-Chloro-9-fluoro-11, 16, 1... |
| Active Ingredient | Halcinonide |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Ranbaxy |
| 2 of 2 | |
|---|---|
| Drug Name | Halog |
| PubMed Health | Halcinonide (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Strong |
| Drug Label | The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include halcinonide. Halcinonide is designated chemically as 21-Chloro-9-fluoro-11, 16, 1... |
| Active Ingredient | Halcinonide |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Ranbaxy |
Indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
FDA Label
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AD - Corticosteroids, very potent (group iv)
D07AD02 - Halcinonide
The precise mechanism of action of topical corticosteroids is unclear. However they possess anti-inflammatory, antipruritic, and vasoconstrictive actions. New research indicates that halcinonide activates MBP (myelin basic protein) expression via smoothened receptor activation. This finding suggests that halcinonide could be used in the treatment of multiple sclerosis therapy as an alternative to Dexamethasone or Methylprednisolone.