


1. Ginipral
2. Gynipral
3. Hexoprenaline
4. Ipradol
5. St 1512
6. St-1512
7. St1512
8. Sulfate, Hexoprenaline
1. Gynipral
2. Bronalin
3. Etoscol
4. 32266-10-7
5. Leanol
6. 30117-45-4
7. Delaprem
8. Hexoprenaline Sulphate
9. Nsc-292266
10. St1512/so4
11. 4,4'-[hexane-1,6-diylbis[imino(1-hydroxyethylene)]]dipyrocatechol Sulphate
12. Dsstox_cid_26688
13. Dsstox_rid_81822
14. Dsstox_gsid_46688
15. Hexoprenaline Sulfate (usan)
16. Hexoprenalinesulphate
17. Hexoprenaline Sulfate [usan]
18. 4,4'-(hexane-1,6-diylbis(imino(1-hydroxyethylene)))dipyrocatechol Sulphate
19. 4-[2-[6-[[2-(3,4-dihydroxyphenyl)-2-hydroxyethyl]amino]hexylamino]-1-hydroxyethyl]benzene-1,2-diol;sulfuric Acid
20. Cas-32266-10-7
21. U851s9102c
22. Ncgc00167522-01
23. Delaprem (tn)
24. Einecs 250-057-2
25. 1,2-benzenediol, 4,4'-(1,6-hexanediylbis(imino(1-hydroxy-2,1-ethanediyl)))bis-, Sulfate (1:1) (salt)
26. Schembl598186
27. Chembl2104813
28. Dtxsid6046688
29. Chebi:31670
30. Hexoprenaline Sulfate [mi]
31. Hexoprenaline Sulfate [jan]
32. St-1512/so4
33. Tox21_112519
34. Nsc292266
35. Hexoprenaline Sulfate [mart.]
36. Hexoprenaline Sulfate [who-dd]
37. Tox21_112519_1
38. Ncgc00167522-02
39. D02396
40. Q27290818
41. 1, 4,4'-[1,6-hexanediylbis[imino(1-hydroxy-2,1-ethanediyl)]]bis-, Sulfate (1:1) (salt)
42. (+/-)-.alpha.,.alpha.'-(hexamethylenebis(iminomethylene))bis(3,4-dihydroxybenzyl Alcohol) Sulfate (1:1) (salt)
43. (+/-)-.alpha.,.alpha.'-(hexamethylenebis(iminomethylene))bis(3,4-dihydroxybenzyl Alcohol) Sulphate (1:1) (salt)
44. 1,2-benzenediol, 4,4'-(1,6-hexanediylbis(imino(1-hydroxy-2,1-ethanediyl)))bis-, Sulphate (1:1) (salt)
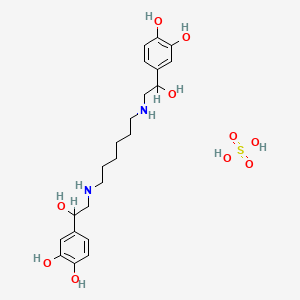
| Molecular Weight | 518.6 g/mol |
|---|---|
| Molecular Formula | C22H34N2O10S |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 13 |
| Exact Mass | 518.19341646 g/mol |
| Monoisotopic Mass | 518.19341646 g/mol |
| Topological Polar Surface Area | 228 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 499 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Adrenergic beta-2 Receptor Agonists
Compounds bind to and activate ADRENERGIC BETA-2 RECEPTORS. (See all compounds classified as Adrenergic beta-2 Receptor Agonists.)
Tocolytic Agents
Drugs that prevent preterm labor and immature birth by suppressing uterine contractions (TOCOLYSIS). Agents used to delay premature uterine activity include magnesium sulfate, beta-mimetics, oxytocin antagonists, calcium channel inhibitors, and adrenergic beta-receptor agonists. The use of intravenous alcohol as a tocolytic is now obsolete. (See all compounds classified as Tocolytic Agents.)