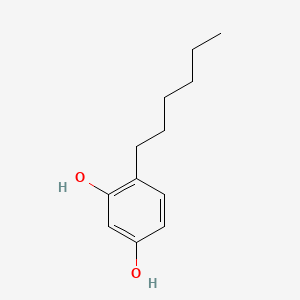

1. 4 Hexylresorcinol
2. 4-hexylresorcinol
1. 4-hexylresorcinol
2. 136-77-6
3. 4-hexylbenzene-1,3-diol
4. 4-hexyl-1,3-benzenediol
5. 4-n-hexylresorcinol
6. Antascarin
7. Ascaricid
8. Ascarinol
9. Oxana
10. P-hexylresorcinol
11. Adrover
12. Caprokol
13. Hidesol
14. Crystoids
15. 1,3-benzenediol, 4-hexyl-
16. Prensol
17. 4-hexylresorcine
18. Sucrets
19. Gelovermin
20. Hexylresorcin
21. 4-hexyl-1,3-dihydroxybenzene
22. Ascaryl
23. 4-(1-hexyl)resorcinol
24. Cystoids Anthelmintic
25. 1,3-dihydroxy-4-hexylbenzene
26. Worm-agen
27. Resorcinol, 4-hexyl-
28. Mycoderm
29. 1,3-dihydroxy-4-n-hexylbenzene
30. Nci-c55787
31. St-37
32. S.t. 37
33. Nsc 1570
34. Mfcd00002284
35. R9qtb5e82n
36. Chembl443605
37. Ins No.586
38. Ins-586
39. Nsc1570
40. 1-(2',4'-dihydroxyphenyl)hexane
41. Ncgc00091208-04
42. Hexylresorzin
43. Dsstox_cid_699
44. Hexylresorcinolum
45. E-586
46. Dsstox_rid_75743
47. Dsstox_gsid_20699
48. Hexylresorcin [german]
49. Everfresh
50. 4-hexyl Resorcinol
51. Cas-136-77-6
52. Ccris 888
53. Hsdb 566
54. St 37
55. Einecs 205-257-4
56. Unii-r9qtb5e82n
57. Brn 2048312
58. Ai3-08055
59. Hexylresorcinol [usp:inn:ban]
60. 4-hexyl-benzenediol
61. Hexylresorcinol (usp)
62. Spectrum_000869
63. 1, 4-hexyl-
64. 4-hexylresorcinol, 8ci
65. Spectrum2_000989
66. Spectrum3_000451
67. Spectrum4_000001
68. Spectrum5_000794
69. 4-hexylresorcinol, 98%
70. Wln: Qr Cq D6
71. Hexylresorcinol, Ban, Usan
72. Schembl29107
73. Bspbio_002122
74. Kbiogr_000341
75. Kbioss_001349
76. 4-06-00-06048 (beilstein Handbook Reference)
77. Mls002152930
78. Divk1c_000094
79. Hexylresorcinol [hsdb]
80. Hexylresorcinol [inci]
81. Spectrum1500330
82. Spbio_001057
83. 4-hexylresorcinol [mi]
84. Hexylresorcinol [mart.]
85. 1-(2,4-dihydroxyphenyl)hexane
86. 4-hexyl-1,3-dihydroxy Benzene
87. 4-hexylresorcinol [fcc]
88. Dtxsid1020699
89. Hexylresorcinol [usp-rs]
90. Hexylresorcinol [who-dd]
91. Bcbcmap01_000093
92. Chebi:93749
93. Hms500e16
94. Kbio1_000094
95. Kbio2_001349
96. Kbio2_003917
97. Kbio2_006485
98. Kbio3_001342
99. Ninds_000094
100. 1, 3-dihydroxy-4-n-hexylbenzene
101. Hms1920d09
102. Hms2091j15
103. Hms3885j08
104. Pharmakon1600-01500330
105. Benzene, 4-hexyl-1,3-dihydroxy-
106. Act05713
107. Bcp31316
108. Hy-b0986
109. Nsc-1570
110. Zinc1576892
111. Tox21_111102
112. Tox21_202263
113. Tox21_300357
114. Bbl023042
115. Bdbm50292636
116. Ccg-40207
117. Hexylresorcinol [ep Impurity]
118. Nsc757056
119. S4571
120. Stl008005
121. Hexylresorcinol [ep Monograph]
122. 4-hexylresorcinol, Analytical Standard
123. Akos005683587
124. Tox21_111102_1
125. Cs-4477
126. Db11254
127. Hexylresorcinol [usp Monograph]
128. Nsc-757056
129. Benzene,1,3-dihydroxy,4-hexyl
130. Idi1_000094
131. Qtl1_000043
132. Smp1_000009
133. Ncgc00091208-01
134. Ncgc00091208-02
135. Ncgc00091208-03
136. Ncgc00091208-05
137. Ncgc00091208-06
138. Ncgc00091208-07
139. Ncgc00091208-09
140. Ncgc00091208-11
141. Ncgc00254302-01
142. Ncgc00259812-01
143. Ac-12698
144. Ac-31726
145. As-40438
146. Nci60_001129
147. Smr000112060
148. Sbi-0051404.p003
149. Ft-0618587
150. H0139
151. D04441
152. Ab00052011_05
153. A807126
154. Q229969
155. Sr-05000002054
156. Q-201203
157. Sr-05000002054-1
158. Brd-k99946902-001-02-6
159. Z1259339944
160. Hexylresorcinol, European Pharmacopoeia (ep) Reference Standard
161. Hexylresorcinol, United States Pharmacopeia (usp) Reference Standard
162. 1-(2',4'-dihydroxyphenyl)hexane;hexylresorcinol;4-hexyl-1,3-benzenediol
163. Hexylresorcinol For System Suitability, Europepharmacopoeia (ep) Reference Standard
| Molecular Weight | 194.27 g/mol |
|---|---|
| Molecular Formula | C12H18O2 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 194.130679813 g/mol |
| Monoisotopic Mass | 194.130679813 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 147 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Local; Antinematodal Agents; Antiplatyhelmintic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
It is commonly employed in 1:1000 soln or glycerite in mouthwashes or pharyngeal antiseptic preparation.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 991
MEDICATION (VET): Rare now, as anthelmintic especially since introduction of dichlorvos and other drugs. Topically it is effective bacteriostatic, bactericidal, virucidal, fungistatic, and fungicidal agent at dilutions greater than 1:1000 (0.1%), although latter is safe topically. In ringworm therapy with Aminoacridinium = Aacrisorcin ...
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 260
MEDICATION (VET): Effective against many viruses when aerosoled at 5 mg/cu m. ... Administer orally in oil to reduce local irritation.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 260
For more Therapeutic Uses (Complete) data for HEXYLRESORCINOL (7 total), please visit the HSDB record page.
Hexylresorcinol should not be dispensed in ordinary, hard-gelatin capsules as these quickly become brittle, and may break in mouth causing caustic burns.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1174
Hexylresorcinol, given orally, is ineffective but when given by enema, tedious and unpleasant experience for patients, there is immediate symptomatic relief, although cures are rarely attained.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 864
Care should be taken that pills containing drug are swallowed whole or painful ulceration of oral mucous membrane may result.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1174
probable oral lethal dose (human) 0.5-5 g/kg, between 1 oz and 1 pint (or 1 lbs) for 70 kg person (150 lb). Somewhat less toxic than resorcinol or phenol.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-127
Hexylresorcinol is predominantly employed as the active ingredient in lotions, sprays, or lozenges indicated as a (a) topical antiseptic to help prevent skin infection in minor cuts, scrapes, or burns, or (b) as an antiseptic and local anesthetic for the relief of a sore throat and its associated pain. In addition, hexylresorcinol is used as an active ingredient in various commercial cosmetic skincare products as an anti-aging cream while other studies have looked into whether or not the compound could be used effectively as an anti-inflammatory agent or even as an anti-cancer therapy.
Hexylresorcinol is a phenol derivative, and in typical therapeutic usage is primarily a local anesthetic for topical use on the mucous membranes of the mouth and throat. The local anesthetic like properties of hexylresorcinol is likely due to its sodium channel blocking effects. The agent also demonstrates mild antiseptic activity as well as an apparent anti-inflammatory, demulcent action.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA12 - Hexylresorcinol
Absorption
Owing to the poor absorption of hexylresorcinol, systemic exposure and symptoms are unusual.
Route of Elimination
When two men received doses of 1 g of hexylresorcinol, an average of 18% of the dose was recovered in the urine within the first 12 hours - thereafter, the compound was not detected in urine samples.
Volume of Distribution
Readily accessible data regarding the volume of distribution of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area.
Clearance
Readily accessible data regarding the clearance of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area.
Dogs were given single doses of 1 or 3 g 4-hexylresorcinol (equivalent to 100 or 300 mg/kg bw) as crystals in gelatin capsules or as a solution in olive oil, and excretion monitored in urine and feces. After administration of 1 g crystalline compound, 29% of the dose was detected in urine and 67% in feces; when the dose was increased to 3 g, 17% was excreted in urine and 73% in feces. Urinary excretion was rapid, mainly in the first 6 hr, and levels were virtually undetectable 12 hr after the lower dose and 24-36 hr following the higher dose. When 4-hexylresorcinol was administered in olive oil, a dose of 1 g resulted in 17% being excreted in urine and 76% in feces, while 10% was excreted in urine and 80% in feces following a dose of 3 g.
WHO Food Additive Series 35: 4-Hexylresorcinol (136-77-6). Available from, as of July 18, 2005: https://www.inchem.org/documents/jecfa/jecmono/v35je04.htm
When two men received doses of 1 g 4-hexylresorcinol, an average of 18% of the dose was recovered in urine within the first 12 hr; thereafter the compound was not detected in urine samples. Fecal excretion accounted for 64% of the dose.
WHO Food Additive Series 35: 4-Hexylresorcinol (136-77-6). Available from, as of July 18, 2005: https://www.inchem.org/documents/jecfa/jecmono/v35je04.htm
Regarding the metabolism of hexylresorcinol, it has been reported that excretion of the compound in the urine is largely in the form of an ethereal sulfate conjugate.
Human metabolite of 4-hexylresorcinol is ethereal sulfate. /From table/
Sunshine, I. (ed.). CRC Handbook of Analytical Toxicology. Cleveland: The Chemical Rubber Co., 1969., p. 353
It has been reported that 4-hexylresorcinol is excreted via the urine mainly in the form of an ethereal sulfate conjugate ...
WHO Food Additive Series 35: 4-Hexylresorcinol (136-77-6). Available from, as of July 18, 2005: https://www.inchem.org/documents/jecfa/jecmono/v35je04.htm
Readily accessible data regarding the half-life of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area.
When acting as an oral anesthetic for relieving sore throats, it is generally believed that hexylresorcinol is possibly capable of blocking voltage-gated neuronal sodium channels, which in turn would inhibit the initiation and conduction of nerve impulses for feeling or transmitting pain signals in the local area to which the hexylresorcinol is applied. As an antiseptic agent, studies have demonstrated that hexylresorcinol is capable of eliciting actions like reducing or inhibiting the generation of bacterial biofilm, interfering with bacterial cell chain formation, reducing bacterial adherence of the pharynx, inhibition of glycolytic enzyme and pH drops, and alteration of cell surface hydrophobicity. Unfortunately, there are either antibiotics that function even more effectively at formally treating bacterial growth or there are also other plant-derived phenolic compounds similar to hexylresorcinol that elicit stronger such mechanisms of action. Nevertheless, it is useful for hexylresorcinol to have both anesthetic and certain antiseptic actions for its use in treating various relatively self-limiting scrapes and sore throats that are treated by the over-the-counter products that feature the compound. Early studies in the 1930s and 1940s suggested that there were more effective medicines over hexylresorcinol that could be employed for their anthelmintic effects. As an anti-inflammatory and anti-aging agent, some studies have shown that it may be possible for hexylresorcinol to inhibit the phosphorylation of the immune response mediator NF-kappaB and also elicit a significant skin lightening effect owing to a strong inhibitory effect on tyrosinase and peroxidase and a stimulatory effect on glutathione and E-cadherin syntheses. It is proposed that hexylresorcinol can bind to tyrosinase directly and inhibits its enzyme activity. Literature data suggests that low glutathione levels relates to the deposition of melanin in the skin of humans and other animals, while high glutathione levels inhibit melanogenesis. And ultimately, it is also reported that glutathione depletion increases tyrosinase activity in human melanoma cells, which makes hexylresorcinol's effects on tyrosinase desirable. Finally, there are ongoing studies that have reported hexylresorcinol's abilities to induce the differentiation of SCC-9 squamous cell cell-line by way of the modulation of the E2F-mediated signaling pathway and suppress the growth of squamous cell carcinoma SCC-9 cells in a dose-dependent manner. Moreover, such studies have also shown that hexylresorcinol is seemingly capable of dose-dependent induction of SCC-9 cell apoptosis as well as the inhibition of transglutaminase-2 enzyme activity which can facilitate chemotherapy resistance.