

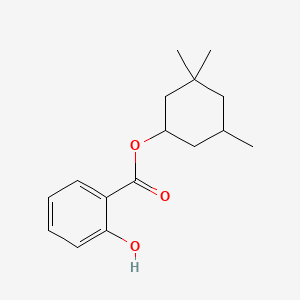

1. 3,3,5-trimethylcyclohexyl Salicylate
1. 118-56-9
2. Homomenthyl Salicylate
3. 3,3,5-trimethylcyclohexyl Salicylate
4. Coppertone
5. Heliopan
6. Heliophan
7. Eusolex
8. Filtersol ''a''
9. (3,3,5-trimethylcyclohexyl) 2-hydroxybenzoate
10. M-homomenthyl Salicylate
11. 3,3,5-trimethylcyclohexyl 2-hydroxybenzoate
12. Homosalatum
13. Benzoic Acid, 2-hydroxy-, 3,3,5-trimethylcyclohexyl Ester
14. Nsc 164918
15. 2-hydroxybenzoic Acid 3,3,5-trimethylcyclohexyl Ester
16. Mfcd00019377
17. Salicylic Acid 3,3,5-trimethylcyclohexyl Ester
18. 52253-93-7
19. Nsc-164918
20. V06sv4m95s
21. Salicylic Acid, 3,3,5-trimethylcyclohexyl Ester
22. Ncgc00091888-01
23. Dsstox_cid_6241
24. Dsstox_rid_78073
25. Dsstox_gsid_26241
26. Salicylic Acid, 3,3,5-trimethylcyclohexyl Ester (8ci)
27. Caswell No. 482b
28. Homosalato
29. 3,3,5-trimethylcyclohexyl Salicylate (cis- And Trans- Mixture)
30. Filtrosol A
31. Homosalatum [inn-latin]
32. Homosalato [inn-spanish]
33. Ccris 4885
34. Component Of Coppertone
35. Sr-05000001884
36. Salicylic Acid, M-homomenthyl Ester
37. Metahomomenthyl Salicylate
38. Einecs 204-260-8
39. Epa Pesticide Chemical Code 076603
40. Unii-v06sv4m95s
41. Homosalat
42. Homosalate [usan:usp:inn]
43. Kemester Hms
44. Cas-118-56-9
45. Homosalate [mi]
46. Homosalate (usp/inn)
47. Homosalate [inn]
48. Prestwick1_001090
49. Prestwick2_001090
50. Prestwick3_001090
51. Homosalate [inci]
52. Homosalate [usan]
53. Ec 204-260-8
54. Homosalate [mart.]
55. Homosalate [usp-rs]
56. Homosalate [who-dd]
57. Schembl16207
58. Bspbio_001140
59. Spectrum1505020
60. Spbio_003030
61. Bpbio1_001254
62. Chembl1377575
63. Dtxsid1026241
64. Chebi:91642
65. Homosalate [usp Impurity]
66. 3,5-trimethylcyclohexyl Salicylate
67. Hms1571i22
68. Hms2093g22
69. Hms2098i22
70. Hms3715i22
71. Homosalate [usp Monograph]
72. Pharmakon1600-01505020
73. Component Of Coppertone (salt/mix)
74. Hy-b0928
75. Tox21_111174
76. Tox21_202109
77. Tox21_303082
78. Nsc164918
79. Nsc758908
80. S4572
81. Akos015904082
82. Tox21_111174_1
83. Ccg-213330
84. Db11064
85. Nsc-758908
86. Ncgc00091888-02
87. Ncgc00091888-03
88. Ncgc00091888-04
89. Ncgc00091888-05
90. Ncgc00091888-06
91. Ncgc00091888-09
92. Ncgc00257063-01
93. Ncgc00259658-01
94. As-10409
95. Sy051923
96. Sbi-0206787.p001
97. Ab00514041
98. Ft-0614020
99. Salicylic Acid,3,5-trimethylcyclohexyl Ester
100. T2278
101. Benzoic Acid, 3,3,5-trimethylcyclohexyl Ester
102. D04450
103. E78223
104. 2,3,3,4,4,5,5,6-octachlorobiphenyl
105. Ab00514041_02
106. A921433
107. J-519754
108. Q2260189
109. Sr-05000001884-1
110. Sr-05000001884-2
111. Brd-a34751532-001-03-6
112. Brd-a34751532-001-04-4
113. 2-hydroxybenzoic Acid (3,3,5-trimethylcyclohexyl) Ester
114. Homosalate, United States Pharmacopeia (usp) Reference Standard
115. 3,3,5-trimethylcyclohexyl Salicylate 100 Microg/ml In Acetonitrile
116. 3,3,5-trimethylcyclohexyl Salicylate 100 Microg/ml In Methanol
117. Homosalate, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 262.34 g/mol |
|---|---|
| Molecular Formula | C16H22O3 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 262.15689456 g/mol |
| Monoisotopic Mass | 262.15689456 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 324 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
As ingredient in many sunscreen for protection against sunburn, skin aging and skin cancer.
Acts as UV filters.
Sunscreening Agents
Chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. (See all compounds classified as Sunscreening Agents.)
Absorption
For local use only, no systemic absorption.
Route of Elimination
For local use only, no systemic absorption.
Volume of Distribution
For local use only, no systemic absorption.
Clearance
For local use only, no systemic absorption.
For local use only, no systemic absorption.
For local use only, no systemic absorption.
Homosalate has the ability to convert incident ultraviolet radiation into less damaging infrared radiation (heat).