


1. Dihydroquinine
2. Hydroquinidine
3. Hydroquinidine Dihydrochloride, (1beta,4beta,3s)-(+-)-isomer
4. Hydroquinidine Dihydrochloride, (3alpha,9s)-(+-)-isomer
5. Hydroquinidine Hydrochloride
6. Hydroquinidine Monosulfate
7. Hydroquinidine Monosulfate, (1beta,3alpha,4beta,8alpha,9r)-isomer
8. Hydroquinidine Monosulfate, (1beta,3alpha,4beta,9s)-isomer
9. Hydroquinidine Sulfate
10. Hydroquinidine Sulfate, (9s)-isomer
11. Hydroquinidine, (+-)-isomer
12. Hydroquinidine, (1beta, 3alpha,4beta,8alpha,9r)-isomer
13. Hydroquinidine, (1beta,3alpha,4beta,9s)-isomer
14. Hydroquinidine, (1beta,4beta,9s)-(+-)-isomer
15. Hydroquinidine, (3alpha,9s)-(+-)-isomer
16. Hydroquinidine, (8alpha,9r)-isomer
17. Hydroquinidine, (8alpha,9s)-isomer
18. Hydroquinidine, (9r)-isomer
19. Hydroquinidine, (9s)-(+-)-isomer
20. Hydroquinine
21. Lcn 834
22. Lcn-834
23. Lentoquine
24. Srcor
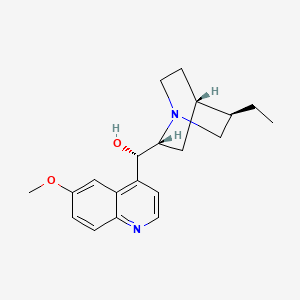
1. Hydroquinidine
2. 1435-55-8
3. Hydroconquinine
4. Hydroconchinine
5. Dihydroquinine
6. (+)-hydroquinidine
7. (9s)-10,11-dihydro-6'-methoxycinchonan-9-ol
8. 8p68xpy4hg
9. Dihydrochinidin
10. 10,11-dihydroquinidine
11. Mfcd00135599
12. Cinchonan-9-ol, 10,11-dihydro-6'-methoxy-, (9s)-
13. (s)-((1s,2r,4s,5r)-5-ethylquinuclidin-2-yl)(6-methoxyquinolin-4-yl)methanol
14. (+)-dihydroquinidine
15. Einecs 215-862-5
16. Gnf-pf-2067
17. Unii-8p68xpy4hg
18. Hydroquinidine, 95%
19. Hydroquinidine [mi]
20. Schembl308961
21. (8r,9s)-10,11-dihydro-6'-methoxy-9-cinchonanol
22. Chembl531472
23. Megxp0_001892
24. Hydroquinidine [who-dd]
25. Acon1_001481
26. Dtxsid50862110
27. Act08913
28. Zinc3977899
29. S4648
30. S4658
31. Ccg-267773
32. Ccg-267774
33. Db15300
34. Nsc 757410
35. Ncgc00180461-01
36. Ncgc00180461-02
37. Ncgc00385753-01
38. (+)-dihydroquinidine, Analytical Standard
39. As-16052
40. A20646
41. Fmoc-(s)-3-amino-4-(2-naphthyl)butanoicacid
42. 435h558
43. Q5276449
44. Brd-k98182306-001-01-3
45. (9s)-10,11-dihydro-6'-methoxycinchonan-9-ol;hydroquinidine
46. (s)-((2r,4s,5r)-5-ethylquinuclidin-2-yl)(6-methoxyquinolin-4-yl)methanol
| Molecular Weight | 326.4 g/mol |
|---|---|
| Molecular Formula | C20H26N2O2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 326.199428076 g/mol |
| Monoisotopic Mass | 326.199428076 g/mol |
| Topological Polar Surface Area | 45.6 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 432 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C - Cardiovascular system
C01 - Cardiac therapy
C01B - Antiarrhythmics, class i and iii
C01BA - Antiarrhythmics, class ia
C01BA13 - Hydroquinidine
