

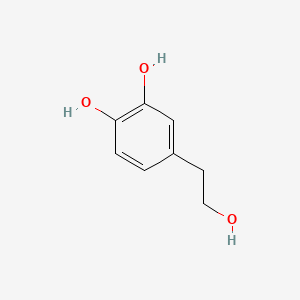

1. 2-(3,4-dihydroxyphenyl)ethanol
2. 3,4-dihydroxyphenylethanol
3. Beta-3,4-dihydroxyphenylethyl Alcohol
4. Dopet
1. 10597-60-1
2. 3,4-dihydroxyphenylethanol
3. 2-(3,4-dihydroxyphenyl)ethanol
4. 4-(2-hydroxyethyl)benzene-1,2-diol
5. Dopet
6. 3,4-dihydroxyphenethyl Alcohol
7. 3-hydroxytyrosol
8. 1,2-benzenediol, 4-(2-hydroxyethyl)-
9. 2-(3,4-dihydroxyphenyl)ethyl Alcohol
10. 3,4-dihydroxyphenethylalcohol
11. 4-(2-hydroxyethyl)-1,2-benzenediol
12. Beta-3,4-dihydroxyphenylethyl Alcohol
13. Dihydroxyphenylethanol
14. Mfcd01320529
15. Qeu0ne4o90
16. Chebi:68889
17. Unii-qeu0ne4o90
18. Dopaol
19. 3,4-dihydroxy-1-benzeneethanol
20. 4-(2-hydroxy-ethyl)-benzene-1,2-diol
21. Hydroxytyrosol [mi]
22. Schembl44363
23. Hydroxytyrosol [inci]
24. 3,4-dhpea
25. Hydroxytyrosol [who-dd]
26. Chembl1950045
27. Ba2774
28. Juubchwrxwpffh-uhfffaoysa-
29. Dtxsid70147451
30. Ba 2774
31. Bcp31094
32. Hy-n0570
33. Zinc2379217
34. 2-(3,4-di-hydroxyphenyl)-ethanol
35. S3826
36. 3-hydroxytyrosol, >=98% (hplc)
37. 3-hydroxytyrosol, Analytical Standard
38. Akos003368868
39. Ac-5308
40. Ccg-266246
41. Db12771
42. As-10017
43. Sy017402
44. Db-008830
45. Am20020163
46. Cs-0009107
47. D2756
48. Ft-0600597
49. Ft-0670206
50. N2302
51. 597d601
52. A801346
53. Q744577
54. Q-100040
55. Brd-k04809113-001-01-7
56. 3,4-dihydroxyphenethyl Alcohol;3,4-dihydroxyphenyl Ethanol
| Molecular Weight | 154.16 g/mol |
|---|---|
| Molecular Formula | C8H10O3 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 154.062994177 g/mol |
| Monoisotopic Mass | 154.062994177 g/mol |
| Topological Polar Surface Area | 60.7 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 116 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)