

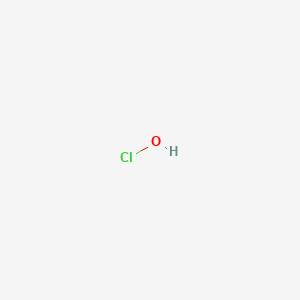

1. Hypochlorite
2. Hypochlorous Acids
1. 7790-92-3
2. Hocl
3. Hclo
4. Chloranol
5. Epicyn Hydrogel
6. Comfosy
7. Hydroxidochlorine
8. Hypochloric Acid
9. Chebi:24757
10. 712k4cdc10
11. Einecs 232-232-5
12. Hydroxychloride
13. Oxylchloride
14. Chloro-alcohol
15. Oxyl Chloride
16. Unii-712k4cdc10
17. Hydroxy Chloride
18. Chlor(i)-saeure
19. Hypochlorige Saeure
20. [cloh]
21. Chlorine Oxide (clo1-)
22. Hypochlorous Acid Ion(1-)
23. Hypochlorous Acid [mi]
24. Chembl1616046
25. Dtxsid3036737
26. Hypochlorous Acid [inci]
27. Hypochlorous Acid [vandf]
28. Hypochlorous Acid [who-dd]
29. Db14135
30. Ft-0695042
31. Ft-0775244
32. C19697
33. Q407318
34. Q2535800
35. 8tr
| Molecular Weight | 52.46 g/mol |
|---|---|
| Molecular Formula | ClHO |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 51.9715923 g/mol |
| Monoisotopic Mass | 51.9715923 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oxidants
Electron-accepting molecules in chemical reactions in which electrons are transferred from one molecule to another (OXIDATION-REDUCTION). (See all compounds classified as Oxidants.)