
1. Ciloprost
2. Ventavis
3. Zk 36374
4. Zk-36374
5. Zk36374
1. Ciloprost
2. Ventavis
3. Endoprost
4. Ilomedin
5. 78919-13-8
6. Iloprostum
7. Zk-36374
8. Jed5k35ygl
9. Zk 36374
10. Chembl494
11. Chebi:63916
12. Zk 00036374
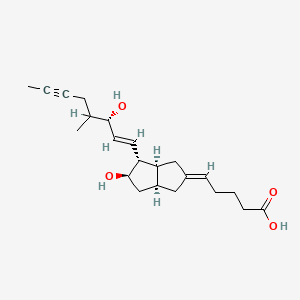
13. (5e)-5-[(3as,4r,5r,6as)-5-hydroxy-4-[(e,3s)-3-hydroxy-4-methyloct-1-en-6-ynyl]-3,3a,4,5,6,6a-hexahydro-1h-pentalen-2-ylidene]pentanoic Acid
14. [3h]-iloprost
15. Ventavis (tn)
16. Zk-00036374
17. 73873-87-7
18. (16r,s)-methyl-18,18,19,19-tetradehydro-6a-carbaprostaglandin I2
19. 15(r)-iloprost
20. (5e)-5-[(3as,4r,5r,6as)-5-hydroxy-4-[(1e,3s)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl]hexahydropentalen-2(1h)-ylidene]pentanoic Acid
21. Iloprostum [latin]
22. Sr-05000001498
23. Unii-jed5k35ygl
24. Iloprost [usan:inn:ban]
25. Ilomedine
26. Bay Q6256
27. Iloprost [usan]
28. Iloprost (usan/inn)
29. Iloprost [inn]
30. Iloprost [jan]
31. Iloprost [mi]
32. Iloprost [vandf]
33. Iloprost [mart.]
34. Iloprost [who-dd]
35. Iloprost [ema Epar]
36. Iloprost [orange Book]
37. Schembl6083382
38. Iloprost, >=98% (hplc)
39. Bay-q-6256
40. Dtxsid2041046
41. Bdbm23954
42. Hms2090a19
43. 85026-51-3
44. Ex-a6213
45. Hy-a0096
46. Sh-401
47. (16r,s)-methyl-18,18,19,19-tetradehydro-6a-carbaprostaglandin I(sub 2)
48. Akos024456922
49. Cs-5586
50. Db01088
51. (e)-5-(3as,4r,5r,6as)-5-hydroxy-4((e)-(3s,4rs)-3-hydroxy-4-methyl-1-octen-6-inyl)perhydropentalen-2-yliden)valeriansaeure
52. Pentanoic Acid, 5-(hexahydro-5-hydroxy-4-(3-hydroxy-4-methyl-1-octen-6-ynyl)-2(1h)-pentalenylidene)-
53. D02721
54. E-1030
55. Aceticacid2-tert-butyl-4-methylphenylester
56. J-502615
57. Sr-05000001498-1
58. Sr-05000001498-2
59. Brd-a45664787-001-01-4
60. Brd-a45664787-001-02-2
61. Q20817139
62. (1s,2r,3r,5s)-7-[(e)-4-carboxybutylidene]-2-[(3s,1e)-3-hydroxy-4-methyl-6-octyne-1-enyl]-3-hydroxybicyclo[3.3.0]octane
63. (5e)-(3as,4r,5r,6as)-5-hydroxy-4-((1e)-(3s,4rs)-3-hydroxy-4-methyloct-1-en-6-ynyl)-hexahydropentalen-2(1h)-ylidene)pentanoic Acid
64. (5e)-5-[(3as,4r,5r,6as)-hexahydro-5-hydroxy-4-[(1e,3s)-3-hydroxy-4-methyl-1-octen-6-ynyl]-2(1h)-pentalenylidene]pentanoic Acid
65. (e)-(3as,4r,5r,6as)-hexahydro-5-hydroxy-4-((e)-(3s,4rs)-3-hydroxy-4-methyl-1-octen-6-ynyl)-delta(sup 2(1h),delta)-pentalenevaleric Acid
66. (z)-5-((3as,4r,5r,6as)-5-hydroxy-4-((3s,e)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl)hexahydropentalen-2(1h)-ylidene)pentanoic Acid
67. 5-[(2e,3as,4r,5r,6as)-5-hydroxy-4-[(1e,3s)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl]-octahydropentalen-2-ylidene]pentanoic Acid
68. Pentanoic Acid, 5-((3as,4r,5r,6as)-hexahydro-5-hydroxy-4-((1e,3s)-3-hydroxy-4-methyl-1-octen-6-ynyl)-2(1h)-pentalenylidene)-, (5e)-
| Molecular Weight | 360.5 g/mol |
|---|---|
| Molecular Formula | C22H32O4 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 360.23005950 g/mol |
| Monoisotopic Mass | 360.23005950 g/mol |
| Topological Polar Surface Area | 77.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the treatment of pulmonary arterial hypertension.
FDA Label
Treatment of patients with primary pulmonary hypertension, classified as New York Heart Association functional class III, to improve exercise capacity and symptoms.
Iloprost is a synthetic analogue of prostacyclin PGI2 that dilates systemic and pulmonary arterial vascular beds. It was shown to affect platelet aggregation, but whether this effect contributes to its vasodilatory action has not been elucidated. There are two diastereoisomers of iloprost and the 4S isomer is reported to exhibit a higher potency in dilating blood vessels compared to the 4R isomer.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B01AC11
B01AC11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC11 - Iloprost
Absorption
Rapidly absorbed with bioavailability of 63%
Volume of Distribution
0.7 to 0.8 L/kg
Clearance
20 mL/min/kg [Normal subjects]
Primarily hepatic. Iloprost is metabolized principally via beta-oxidation of the carboxyl side chain.
20-30 minutes
Iloprost is a second generation structural analog of prostacyclin (PGI) with about ten-fold greater potency than the first generation stable analogs, such as carbaprostacyclin. Iloprost binds with equal affinity to human prostacyclin (Prostanoid IP) and prostaglandin EP1 receptors. Iloprost constricts the ilium and fundus circular smooth muscle as strongly as prostaglandin E2 (PGE2) itself. Iloprost inhibits the ADP, thrombin, and collagen-induced aggregation of human platelets. In whole animals, iloprost acts as a vasodilator, hypotensive, antidiuretic, and prolongs bleeding time. All of these properties help to antagonize the pathological changes that take place in the small pulmonary arteries of patients with pulmonary hypertension.