

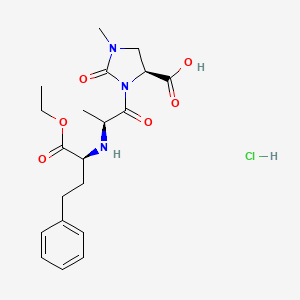

1. 1-methyl-3-(2-(n-(1-ethoxycarbonyl-3-phenylpropyl)amino)propionyl)-2-oxoimidazolidine-4-carboxylic Acid
2. 4-imidazolidinecarboxylic Acid, 3-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1-methyl-2-oxo-, Monohydrochloride, (4s-(3(r*(r*)),4r*))-
3. Imidapril
4. Imidapril Hcl
5. Ta 6366
6. Ta-6366
7. Tanatril
1. 89396-94-1
2. Imidapril Hcl
3. Imidapril (hydrochloride)
4. Ta-6366
5. Imidapril Monohydrochloride
6. Imidapril Hydrochloride [jan]
7. Ta 6366
8. Tanatril
9. 7nsf9gg1nu
10. Novaloc
11. (4s)-3-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-1-methyl-2-oxoimidazolidine-4-carboxylic Acid;hydrochloride
12. 4-imidazolidinecarboxylic Acid, 3-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1-methyl-2-oxo-, Monohydrochloride, (4s-(3(r*(r*)),4r*))-
13. Dsstox_cid_26912
14. Dsstox_rid_82010
15. Dsstox_gsid_46912
16. (4s)-3-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-1-methyl-2-oxoimidazolidine-4-carboxylic Acid,hydrochloride
17. 4-imidazolidinecarboxylic Acid, 3-((2s)-2-(((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1-methyl-2-oxo-, Monohydrochloride, (4s)-
18. Tanapril
19. (s)-3-((s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanoyl)-1-methyl-2-oxoimidazolidine-4-carboxylic Acid Hydrochloride
20. Cas-89396-94-1
21. Ncgc00181342-01
22. Unii-7nsf9gg1nu
23. Novarok
24. Tanatril (tn)
25. Imidapril Hydrochloride,(s)
26. Palmiticacidpropylester
27. Mls004712066
28. Schembl136722
29. Chembl3183293
30. Dtxsid7046912
31. Imidapril Hydrochloride (jp17)
32. Chebi:31691
33. Hy-b1451
34. Tox21_112801
35. Ac-526
36. Mfcd00923828
37. S2109
38. Akos015994537
39. Tox21_112801_1
40. Bs-1005
41. Ccg-269148
42. Cs-5010
43. Imidapril Hydrochloride [mart.]
44. Sh-6366
45. Imidapril Hydrochloride [who-dd]
46. Imidapril Monohydrochloride [mi]
47. Ncgc00181342-02
48. (4s)-3-[(2s)-2-[[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1-methyl-2-oxo-4-imidazolidinecarboxylic Acid Hydrochloride
49. Imidapril Hydrochloride, >=98% (hplc)
50. Smr003475023
51. I0901
52. C77009
53. D01549
54. A917212
55. Q27268625
56. (s)-3-((s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)propanoyl)-1-methyl-2-oxoimidazolidine-4-carboxylicacidhydrochloride
57. (s)-3-((s)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl)-1-methyl-2-oxoimidazolidine-4-carboxylic Acid Hydrochloride
58. 3-((2s)-2-((1s)-n-(1-ethoxycarbonyl-3-phenylpropyl)amino)-propionyl)-1-methyl-2-oxoimidazolidine-4-carboxylic Acid, Hydrochloride-, 4s-
59. 4-imidazolidinecarboxylic Acid, 3-((2s)-2-(((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1-methyl-2-oxo-, Hydrochloride (1:1), (4s)-
| Molecular Weight | 441.9 g/mol |
|---|---|
| Molecular Formula | C20H28ClN3O6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Exact Mass | 441.1666633 g/mol |
| Monoisotopic Mass | 441.1666633 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 619 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)