

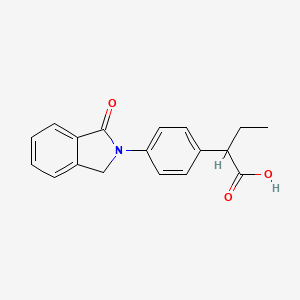

1. 2-(4-(1-oxo-2-isoindolinyl)phenyl)butyric Acid
2. Ibustrin
3. Indobufen, (+-)-isomer
4. K 3920
1. 63610-08-2
2. Ibustrin
3. Indobufen [inn]
4. K-3920
5. 2-[4-(3-oxo-1h-isoindol-2-yl)phenyl]butanoic Acid
6. 6t9949g4lz
7. Indobufen (inn)
8. 2-(4-(1-oxoisoindolin-2-yl)phenyl)butanoic Acid
9. Benzeneacetic Acid, 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-alpha-ethyl-
10. (2s)-2-[4-(3-oxo-1h-isoindol-2-yl)phenyl]butanoic Acid
11. Benzeneacetic Acid, 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-.alpha.-ethyl-
12. K-2930
13. Indobufene
14. Indobufenum
15. K 3920
16. Indobufene [inn-french]
17. Indobufenum [inn-latin]
18. 2-(4-(1-oxo-2-isoindolinyl)phenyl)butyric Acid
19. (+/-)-2-[p-(1-oxo-2-isoindolinyl)phenyl]butyric Acid
20. Unii-6t9949g4lz
21. (+/-)-2-(p-(1-oxo-2-isoindolinyl)phenyl)butyric Acid
22. 36690-96-7
23. Einecs 264-364-4
24. (+/-)-indobufen
25. Enamine_001568
26. Indobufen [mi]
27. Indobufen [mart.]
28. Indobufen [who-dd]
29. Dsstox_cid_31578
30. Dsstox_rid_97463
31. Dsstox_gsid_57789
32. Oprea1_118499
33. Schembl140517
34. (+-)-2-(4-(1-oxo-2-isoindolinyl)phenyl)buttersaeure
35. Chembl1765292
36. Dtxsid7057789
37. 1-oxo-2-(p-((alpha-ethyl)carboxymethyl)phenyl)isoindoline
38. Chebi:135239
39. (+-)-2-(4-(1,3-dihydro-1-oxo-2-isoindolyl)buttersaeure
40. Hms1398h06
41. Amy33419
42. Bcp04485
43. Tox21_113871
44. Ac-672
45. Mfcd00572250
46. S5019
47. 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-alpha-ethyl-benzeneacetic Acid
48. Akos015917718
49. Ccg-267421
50. Db12545
51. Ks-5220
52. Sb66092
53. Ncgc00253758-01
54. Hy-18763
55. Cas-63610-08-2
56. Cs-0014291
57. Ft-0616451
58. D07141
59. D70894
60. 610i082
61. A851741
62. J-521529
63. Q3798322
64. (+-)-2-(p-(1-oxo-2-isoindolinyl)phenyl)butyric Acid
65. 2-(4-(1-carboxypropyl)phenyl)-1-isoindolinone
66. Butyric Acid, 2-(p-(1-oxo-2-isoindolinyl)phenyl)-, (+-)-
67. 1-oxo-2-(p-((.alpha.-ethyl)carboxymethyl)phenyl)isoindoline
68. 4-(1,3-dihydro-1-oxo-(2h)-isoindol-2-yl)-alpha-ethylbenzeneacetic Acid
69. 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-.alpha.-ethylbenzeneacetic Acid
70. Benzeneacetic Acid, 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-alpha-ethyl-, (+-)-
71. Benzeneacetic Acid, 4-(1,3-dihydro-1-oxo-2h-isoindol-2-yl)-.alpha.-ethyl-, (+/-)-
| Molecular Weight | 295.3 g/mol |
|---|---|
| Molecular Formula | C18H17NO3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 295.12084340 g/mol |
| Monoisotopic Mass | 295.12084340 g/mol |
| Topological Polar Surface Area | 57.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 428 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC10 - Indobufen
