


1. Cardio Green
2. Cardio-green
3. Green, Indocyanine
4. Indocyanine Green
5. Ujoveridin
6. Vofaverdin
7. Vophaverdin
8. Wofaverdin
1. Indocyanine Green
2. 3599-32-4
3. Cardio-green
4. Foxgreen
5. Mfcd00013078
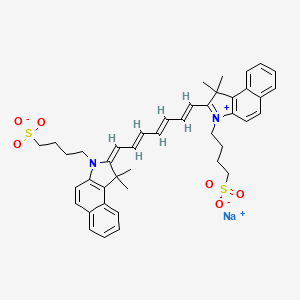
6. Sodium;4-[(2e)-2-[(2e,4e,6e)-7-[1,1-dimethyl-3-(4-sulfonatobutyl)benzo[e]indol-3-ium-2-yl]hepta-2,4,6-trienylidene]-1,1-dimethylbenzo[e]indol-3-yl]butane-1-sulfonate
7. Indocyaninegreen
8. Sodium 4-(2-(7-(1,1-dimethyl-3-(4-sulfonatobutyl)-1h-benzo[e]indol-2(3h)-ylidene)hepta-1,3,5-trien-1-yl)-1,1-dimethyl-1h-benzo[e]indol-3-ium-3-yl)butane-1-sulfonate
9. 1h-benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2h-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-, Inner Salt, Sodium Salt
10. Cardiogreen, For Microscopy
11. Cardiogreen, Polymethine Dye
12. Schembl16027582
13. Akos015896404
14. Akos016010308
15. As-11023
16. I0535
17. A823083
18. Q905662
19. Indocyanine Green, United States Pharmacopeia (usp) Reference Standard
20. Sodium 4-[(2e)-2-[(2e,4e,6e)-7-[1,1-dimethyl-3-(4-sulfonatobutyl)-2-benzo[e]indol-3-iumyl]hepta-2,4,6-trienylidene]-1,1-dimethyl-3-benzo[e]indolyl]-1-butanesulfonate
21. Sodium 4-[(2e)-2-[(2e,4e,6e)-7-[1,1-dimethyl-3-(4-sulfonatobutyl)benzo[e]indol-3-ium-2-yl]hepta-2,4,6-trienylidene]-1,1-dimethyl-benzo[e]indol-3-yl]butane-1-sulfonate
| Molecular Weight | 775.0 g/mol |
|---|---|
| Molecular Formula | C43H47N2NaO6S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 12 |
| Exact Mass | 774.27732385 g/mol |
| Monoisotopic Mass | 774.27732385 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1520 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Ic-green |
| Drug Label | ICGREEN is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green. IC... |
| Active Ingredient | Indocyanine green |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/vial |
| Market Status | Prescription |
| Company | Akorn |
| 2 of 4 | |
|---|---|
| Drug Name | Indocyanine green |
| Drug Label | ICGREEN is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green. IC... |
| Active Ingredient | Indocyanine green |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/vial |
| Market Status | Prescription |
| Company | Pulsion Medcl |
| 3 of 4 | |
|---|---|
| Drug Name | Ic-green |
| Drug Label | ICGREEN is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green. IC... |
| Active Ingredient | Indocyanine green |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/vial |
| Market Status | Prescription |
| Company | Akorn |
| 4 of 4 | |
|---|---|
| Drug Name | Indocyanine green |
| Drug Label | ICGREEN is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5% sodium iodide. It is packaged with Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green. IC... |
| Active Ingredient | Indocyanine green |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/vial |
| Market Status | Prescription |
| Company | Pulsion Medcl |
Dyes
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
TO DETERMINE CARDIAC OUTPUT, HEPATIC FUNCTION, & LIVER BLOOD FLOW. FOR HEPATIC FUNCTION STUDIES, CALCULATED AMT OF DIAGNOSTIC AGENT IS INJECTED INTO ARM VEIN. 20 MIN AFTER INJECTION, 6 ML OF VENOUS BLOOD IS WITHDRAWN FROM OPPOSITE ARM. AFTER COAGULATION & CENTRIFUGATION, CLEAR SERUM IS READ IN PHOTOMETER @ 800-810 NM.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
DYE RETENTION OF LESS THAN 4% IS FOUND IN HEALTHY SUBJECTS. ... FAILURE TO REMOVE DYE, AS INDICATED BY SERUM LEVELS IN EXCESS OF 4%, IS INDICATIVE OF IMPAIRED HEPATIC FUNCTION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
USUAL, IV, BLOOD VOL DETERMINATION, 5 MG IN 1 ML; HEPATIC FUNCTION DETERMINATION, 0-5 MG/KG.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
DYE IS STABLE IN PLASMA & WHOLE BLOOD PERMITTING LATER ANALYSIS. USERS VARY BOTH CONCN & DOSAGE. IN MAN TOTAL DOSAGE HAS NORMALLY BEEN BELOW 2 MG/KG.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 275
...CONTAINS SMALL AMT OF /5%/ SODIUM IODIDE; THUS, IT SHOULD BE USED WITH CAUTION IN PT ALLERGIC TO IODIDES & RADIOACTIVE IODINE UPTAKE STUDIES SHOULD NOT BE PERFORMED FOR AT LEAST 1 WK FOLLOWING ITS USE. SINCE PROBENECID HAS BEEN SHOWN IN DOGS TO AFFECT HEPATIC UPTAKE, THIS POSSIBILITY SHOULD BE KEPT IN MIND.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
SAFE USE OF THIS DRUG IN PREGNANCY HAS NOT BEEN ESTABLISHED.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
USE FRESH SOLN ONLY AS RECOMMENDED.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 275
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CX - Other diagnostic agents
V04CX01 - Indocyanine green
FOLLOWING IV INJECTION, INDOCYANINE GREEN IS RAPIDLY BOUND TO PLASMA PROTEIN, QUICKLY REMOVED FROM CIRCULATION BY LIVER, & EXCRETED IN BILE IN UNCONJUGATED FORM.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1214
WITH INDOCYANINE GREEN, IT HAS BEEN SHOWN THAT RAT HAS HIGHER MAX BILIARY EXCRETION RATE (0.065 MG/KG/MIN) THAN DOES RABBIT (0.05 MG/KG/MIN) OR DOG (0.027 MG/KG/MIN).
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 139
T/2 INCR WITH INCR DOSAGE. ... CONSIDERABLE DIFFERENCE HAS BEEN NOTED BETWEEN NORMAL MONGRELS & PUREBRED BEAGLES IN CLEARANCE TIMES.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 275
APPARENT HEPATIC EXTRACTION OF INDOCYANINE GREEN IN RATS WAS SURPRISINGLY LOW @ DOSES ABOVE 1 MG/KG. CHANGES IN HEPATIC BLOOD FLOW WOULD NOT ALTER CLEARANCE.
PMID:512932 IGA T, KLAASSEN CD; J PHARMACOL EXP THER 211 (3): 690 (1979)
For more Absorption, Distribution and Excretion (Complete) data for INDOCYANINE GREEN (10 total), please visit the HSDB record page.
...INDOCYANINE GREEN...APPEARS IN BILE UNCHANGED
Patty, F. (ed.). Industrial Hygiene and Toxicology: Volume II: Toxicology. 2nd ed. New York: Interscience Publishers, 1963., p. 436