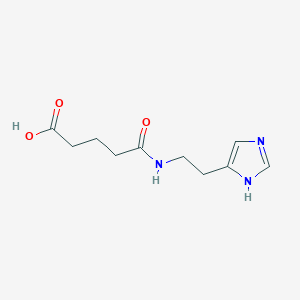

1. 5-(2-(1h-imidazol-4-yl)ethylamino)-5-oxopentanoic Acid
2. Dicarbamin
3. Imidazolyl Ethanamide Pentandioic Acid
4. Pentanedioic Acid Imidazolyl Ethanamide
5. Vitaglutam
1. 219694-63-0
2. Ingamine
3. Imidazolyl Ethanamide Pentandioic Acid
4. Pentanedioic Acid Imidazolyl Ethanamide
5. 4-{[2-(1h-imidazol-5-yl)ethyl]carbamoyl}butanoic Acid
6. 3cm03muj69
7. Pentanoic Acid, 5-[[2-(1h-imidazol-4-yl)ethyl]amino]-5-oxo-
8. 4-[2-(1h-imidazol-4-yl)-ethylcarbamoyl]-butyric Acid
9. Dicarbamin
10. Vitaglutam
11. Pentanoic Acid, 5-((2-(1h-imidazol-4-yl)ethyl)amino)-5-oxo-
12. Agn-pc-0mw29y
13. 4-([2-(1h-imidazol-5-yl)ethyl]carbamoyl)butanoic Acid
14. 5-[2-(1h-imidazol-5-yl)ethylamino]-5-oxopentanoic Acid
15. Unii-3cm03muj69
16. Schembl8282926
17. Chembl4297291
18. Schembl18381264
19. 5-(2-(1h-imidazol-4-yl)ethylamino)-5-oxopentanoic Acid
20. Dtxsid90433141
21. Zinc40493529
22. Akos002807987
23. Db11944
24. D11250
25. F75163
26. Imidazolyl Ethanamide Pentandioic Acid [who-dd]
27. Q27257036
28. 4-{[2-(1h-imidazol-5-yl)ethyl]carbamoyl}butanoicacid
29. 6-(2-(1h-imidazol-4-yl)ethylamino)-5-oxohexanoic Acid
30. 5-((2-(1h-imidazol-5-yl)ethyl)amino)-5-oxopentanoic Acid
| Molecular Weight | 225.24 g/mol |
|---|---|
| Molecular Formula | C10H15N3O3 |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 225.11134135 g/mol |
| Monoisotopic Mass | 225.11134135 g/mol |
| Topological Polar Surface Area | 95.1 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX21 - Pentanedioic acid imidazolyl ethanamide