
1. Optiray
2. Optiray 300
3. Optiray 320
1. 87771-40-2
2. Optiray
3. Mp-328
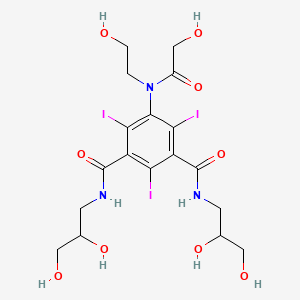
4. 1,3-benzenedicarboxamide, N,n'-bis(2,3-dihydroxypropyl)-5-((hydroxyacetyl)(2-hydroxyethyl)amino)-2,4,6-triiodo-
5. Mp 328
6. Loversol
7. 1-n,3-n-bis(2,3-dihydroxypropyl)-5-[(2-hydroxyacetyl)-(2-hydroxyethyl)amino]-2,4,6-triiodobenzene-1,3-dicarboxamide
8. N3rib7x24k
9. Optiray 320
10. N,n'-bis(2,3-dihydroxypropyl)-5-(n-(2-hydroxyethyl)glycolamido)-2,4,6-triiodoisophthalamide
11. Dsstox_cid_25521
12. Dsstox_rid_80927
13. Dsstox_gsid_45521
14. Ioversolum [latin]
15. Optiray 160
16. Optiray 240
17. Optiray 300
18. Optiray 350
19. Ioversolum
20. Unii-n3rib7x24k
21. Brn 7155654
22. Ioversol [usan:usp:inn:ban]
23. Ncgc00016956-01
24. Optiray (tn)
25. Cas-87771-40-2
26. Ioversol [usan]
27. Ioversol [inn]
28. Ioversol [jan]
29. Ioversol [mi]
30. Ioversol [vandf]
31. Prestwick0_000878
32. Prestwick1_000878
33. Prestwick2_000878
34. Prestwick3_000878
35. Ioversol [mart.]
36. Ioversol [usp-rs]
37. Ioversol [who-dd]
38. Ioversol (jan/usp/inn)
39. Schembl24711
40. Bspbio_000955
41. Spbio_002876
42. Ioversol [orange Book]
43. Bpbio1_001051
44. Chembl1200614
45. Dtxsid2045521
46. Ioversol [usp Monograph]
47. Chebi:31717
48. Hms1570p17
49. Hms2097p17
50. Hms3714p17
51. Pharmakon1600-01503837
52. Bcp11109
53. Hy-b1410
54. Tox21_110709
55. Ac-536
56. Mp-328mp-328
57. Nsc760064
58. S5013
59. Akos015896385
60. Tox21_110709_1
61. Ccg-213209
62. Cs-7483
63. Db09134
64. Nsc-760064
65. Ncgc00179364-01
66. Ncgc00179364-03
67. 8771-40-2
68. As-12851
69. Ab00513943
70. Ft-0627284
71. D01555
72. Ab00513943_02
73. 771i402
74. Sr-01000872680
75. Q-201247
76. Q6064187
77. Sr-01000872680-1
78. Brd-a65818372-001-01-2
79. N,n'-bis (2,3-dihydroxypropyl)-5-[n-(2-hydroxyethyl) -glycolamido] -2,4,6-triiodoisophthalamide
80. N1,n3-bis(2,3-dihydroxypropyl)-5-(2-hydroxy-n-(2-hydroxyethyl)acetamido)-2,4,6-triiodoisophthalamide
| Molecular Weight | 807.1 g/mol |
|---|---|
| Molecular Formula | C18H24I3N3O9 |
| XLogP3 | -3 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 12 |
| Exact Mass | 806.8647 g/mol |
| Monoisotopic Mass | 806.8647 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 623 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Optiray 240 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 2 of 8 | |
|---|---|
| Drug Name | Optiray 300 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 64% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 3 of 8 | |
|---|---|
| Drug Name | Optiray 320 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 68% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 4 of 8 | |
|---|---|
| Drug Name | Optiray 350 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 74% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 5 of 8 | |
|---|---|
| Drug Name | Optiray 240 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 51% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 6 of 8 | |
|---|---|
| Drug Name | Optiray 300 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 64% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 7 of 8 | |
|---|---|
| Drug Name | Optiray 320 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 68% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 8 of 8 | |
|---|---|
| Drug Name | Optiray 350 |
| Drug Label | Optiray (ioversol injection) formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular administration as diagnostic radiopaque media. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) |
| Active Ingredient | Ioversol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 74% |
| Market Status | Prescription |
| Company | Mallinckrodt |
Optiray 350 is indicated in adults for peripheral and coronary arteriography and left ventriculography. Optiray 350 is also indicated for contrast enhanced computed tomographic imaging of the head and body, intravenous excretory urography, intravenous digital subtraction angiography and venography. Optiray 350 is indicated in children for angiocardiography. Optiray 320 is indicated in adults for angiography throughout the cardiovascular system. The uses include cerebral, coronary, peripheral, visceral and renal arteriography, venography, aortography, and left ventriculography. Optiray 320 is also indicated for contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography. Optiray 320 is indicated in children for angiocardiography, contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography. Optiray 300 is indicated for cerebral angiography and peripheral arteriography. Optiray 300 is also indicated for contrast enhanced computed tomographic imaging of the head and body, venography, and intravenous excretory urography. Optiray 240 is indicated for cerebral angiography and venography. Optiray 240 is also indicated for contrast enhanced computed tomographic imaging of the head and body and intravenous excretory urography.
FDA Label
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08AB07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB07 - Ioversol
Absorption
Ioversol may be visualized in the renal parenchyma within 30 to 60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1 to 3 minutes, with optimum contrast occurring within 5 to 15 minutes.
Route of Elimination
Ioversol is excreted mainly through the kidneys following intravascular administration. Greater than 95% of the administered dose was excreted within the first 24 hours, with the peak urine concentration occurring in the first 2 hours after administration. Fecal elimination was negligible.
No significant metabolism, deiodination or biotransformation occurs.
1.5 hr
Intravascular injection of ioversol opacifies those vessels in the path of the flow of the contrast medium, permitting radiographic visualization of the internal structures until significant hemodilution occurs. Optiray enhances computed tomographic imaging through augmentation of radiographic efficiency with the degree of density enhancement directly related to the iodine content in an administered dose.