

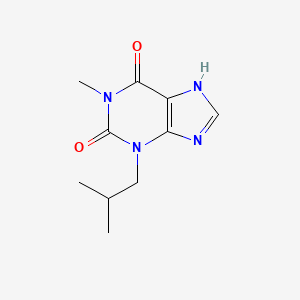

1. 1 Methyl 3 Isobutylxanthine
2. 1-methyl-3-isobutylxanthine
3. 3 Isobutyl 1 Methylxanthine
4. Ibmx
5. Isobutyltheophylline
1. Ibmx
2. 28822-58-4
3. Isobutylmethylxanthine
4. 1-methyl-3-isobutylxanthine
5. Methylisobutylxanthine
6. 3-isobutyl-1-methylxanthine (ibmx)
7. 1h-purine-2,6-dione, 3,7-dihydro-1-methyl-3-(2-methylpropyl)-
8. Xanthine, 3-isobutyl-1-methyl-
9. 3-isobutyl-1-methyl-1h-purine-2,6(3h,7h)-dione
10. 3-isobutyl-1-methyxanthine
11. 1-methyl-3-(2-methylpropyl)-7h-purine-2,6-dione
12. 3-isobutyl-1-methyl-3,9-dihydro-1h-purine-2,6-dione
13. 3-isobutyl-1-methyl-7h-xanthine
14. 3,7-dihydro-3-isobutyl-1-methyl-1h-purine-2,6-dione
15. Nsc 165960
16. Chebi:34795
17. C10h14n4o2
18. 3-isobutyl-1-methyl-3,7-dihydro-1h-purine-2,6-dione
19. 2-acetoxy-benzoic Acid
20. 3-isobutyl-methylxanthine
21. Nsc165960
22. 1-methyl-3-(2-methylpropyl)-3,7-dihydro-1h-purine-2,6-dione
23. 3-isobutyl-1-methyl-xanthine
24. Chembl275084
25. Tbt296u68m
26. Nsc-165960
27. Sc 2964
28. 1-methyl-3-(2-methylpropyl)-3,9-dihydro-1h-purine-2,6-dione
29. 3,7-dihydro-1-methyl-3-(2-methylpropyl)-1h-purine-2,6-dione
30. Methyl-isobutylxanthine
31. 3-isobutyl 1-methylxanthine
32. Smr000326697
33. Ccris 4290
34. Sr-01000075185
35. Einecs 249-259-3
36. Mfcd00005584
37. Unii-tbt296u68m
38. 1zkl
39. 1zkn
40. 3ecn
41. 3itu
42. 3jwr
43. 2hd1
44. 2r8q
45. 3qi4
46. Spectrum2_001705
47. Spectrum2_001735
48. Spectrum3_001958
49. Spectrum4_001052
50. Spectrum5_001856
51. Lopac-i-5879
52. Molmap_000030
53. 3-isobutyl-1-methylanxthine
54. Lopac0_000642
55. Oprea1_135287
56. Oprea1_228781
57. Schembl50315
58. 3-isobutyl-1-methylxantliine
59. Bspbio_001153
60. Bspbio_003558
61. Gtpl388
62. Kbiogr_000493
63. Kbiogr_001344
64. Kbiogr_002566
65. Kbioss_000493
66. Kbioss_002575
67. 1-methyl-3-isobutyl-xanthine
68. Mls001056732
69. Mls001066424
70. Divk1c_000922
71. Spectrum1505298
72. Spectrum2300204
73. Spbio_001690
74. Spbio_001810
75. Dtxsid0040549
76. Bcbcmap01_000110
77. Bdbm15336
78. Chebi:43253
79. Hms502o04
80. Kbio1_000922
81. Kbio2_000493
82. Kbio2_002566
83. Kbio2_003061
84. Kbio2_005134
85. Kbio2_005629
86. Kbio2_007702
87. Kbio3_000905
88. Kbio3_000906
89. Kbio3_002878
90. Kbio3_003044
91. 3-isobutyl-1-methyl-9h-xanthine
92. Cmap_000087
93. Ninds_000922
94. 1-methyl-3-(2-methylpropyl)-1,3,7-trihydropurine-2,6-dione
95. Bio1_000456
96. Bio1_000945
97. Bio1_001434
98. Bio2_000407
99. Bio2_000887
100. Hms1362i15
101. Hms1792i15
102. Hms1990i15
103. Hms2090j10
104. Hms2231c11
105. Hms3262a05
106. Hms3369e16
107. Hms3403i15
108. Hms3604d14
109. Hms3648o18
110. Albb-024315
111. Bcp13248
112. Zinc3861807
113. Tox21_500642
114. 3-isobuthyl-1-methylxanthine
115. Bdbm50027176
116. Ccg-39513
117. Ccg-39624
118. Hsci1_000261
119. Pdsp1_000324
120. Pdsp2_000322
121. S5836
122. Stl558248
123. Akos003390599
124. Akos015903085
125. Cs-3361
126. Db07954
127. Lp00642
128. Sc-2964
129. Sdccgsbi-0050622.p003
130. Idi1_000922
131. Idi1_002162
132. Ncgc00015559-01
133. Ncgc00015559-02
134. Ncgc00015559-03
135. Ncgc00015559-04
136. Ncgc00015559-05
137. Ncgc00015559-06
138. Ncgc00015559-07
139. Ncgc00015559-08
140. Ncgc00015559-09
141. Ncgc00015559-10
142. Ncgc00015559-11
143. Ncgc00015559-21
144. Ncgc00094009-01
145. Ncgc00094009-02
146. Ncgc00094009-03
147. Ncgc00094009-04
148. Ncgc00094009-05
149. Ncgc00094009-06
150. Ncgc00261327-01
151. Xanthine, 1-methyl-3-(2-methylpropyl)
152. As-71134
153. Bi164529
154. Hy-12318
155. Ibmx(nsc165960; Sc2964)
156. 3-isobutyl-1-methyl-7h-purine-2,6-dione
157. Db-047466
158. [eur J Pharmacol 170: 35 (1989)]
159. B7206
160. Eu-0100642
161. Ft-0615920
162. Wln: T56 Bm Dn Fnvnvj F1y1&1 H1
163. D71221
164. I 5879
165. 3-isobutyl-1-methylxanthine, Bioultra, >=99%
166. 822i584
167. A876607
168. L001156
169. Q223093
170. 3-isobutyl-1-methylxanthine - Cas 28822-58-4
171. J-640140
172. J-800144
173. Sr-01000075185-1
174. Sr-01000075185-6
175. 3-isobutyl-1-methylxanthine, >=99% (hplc), Powder
176. Brd-k94979336-001-06-9
177. Brd-k94979336-001-09-3
178. 1h-purine-2, 3,7-dihydro-1-methyl-3-(2-methylpropyl)-
179. Imx;isobutylmethylxanthine;methylisobutylxanthine;nsc165960;sc2964
180. 1-methyl-3-(2-methylpropyl)-2,3,6,7-tetrahydro-1h-purine-2,6-dione
181. 1-methyl-3-(2-methylpropyl)-2,3,6,9-tetrahydro-1h-purine-2,6-dione
| Molecular Weight | 222.24 g/mol |
|---|---|
| Molecular Formula | C10H14N4O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 222.11167570 g/mol |
| Monoisotopic Mass | 222.11167570 g/mol |
| Topological Polar Surface Area | 69.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 318 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
