

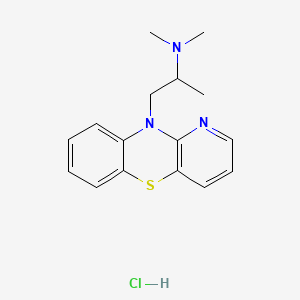

1. 10-(2-dimethylamino-2-methylethyl)-10h-pyrido(3,2- B)(1,4)benzothiazine
2. Andantol
3. Andanton
4. Estamyl
5. Isothipendyl
6. Sedermyl
1. 1225-60-1
2. Andantol
3. Theruhistin
4. Adantol
5. Isothipendyl Hcl
6. Isothipendyl Monohydrochloride
7. Isothipendyl Hydrochloride [jan]
8. Andanton
9. Nilergex
10. Nsc 169186
11. Nsc-169186
12. Isothipendyl (hydrochloride)
13. 10-(2-dimethylaminopropyl)-10h-pyrido(3,2-b)(1,4)benzothiazine Hydrochloride
14. 953ap1lbv8
15. N-dimethylaminoisopropylthiophenylpyridylamine Hydrochloride
16. 34433-15-3
17. Nsc169186
18. (+/-)-isothipendyl Hydrochloride
19. 10h-pyrido(3,2-b)(1,4)benzothiazine-10-ethanamine, N,n,2-trimethyl-, Monohydrochloride
20. N,n-dimethyl-1-pyrido[3,2-b][1,4]benzothiazin-10-ylpropan-2-amine;hydrochloride
21. Adanton Hydrochloride
22. Udantol Hydrochloride
23. Nilergex Hydrochloride
24. Isothipendyl Hydrochloride (jan)
25. Theruhistin Hydrochloride
26. Einecs 214-957-9
27. Unii-953ap1lbv8
28. Andantol (tn)
29. Einecs 252-022-7
30. 10-(2-dimethylaminopropyl)-1-azaphenothiazine Hydrochloride
31. Dimethylamino-isopropyl-thiophenyl-pyridylamin Hydrochlorid [german]
32. Ncgc00181101-01
33. 10-(2-dimethylaminopropyl)-(1)-4-azaphenthiazin Hydrochlorid [german]
34. 10-(2-dimethylaminopropyl)-9-thia-1,10-diazaanthracene Hydrochloride
35. 10-(2-dimethylamino-2-methylethyl)-10h-pyrido(3,2-b)(1,4)benzothiazine Hydrochloride
36. 10h-pyrido(3,2-b)(1,4)benzothiazine, 10-(2-(dimethylamino)propyl)-, Monohydrochloride
37. Dsstox_cid_26845
38. Dsstox_rid_81955
39. Dsstox_gsid_46845
40. Schembl230214
41. Chembl2107246
42. Dtxsid1046845
43. Chebi:31734
44. Dimethylamino-isopropyl-thiophenyl-pyridylamin Hydrochlorid
45. 10-(2-dimethylaminopropyl)-(1)-4-azaphenthiazin Hydrochlorid
46. Tox21_112717
47. Isothipendyl Hydrochloride [mi]
48. 10-(2-(dimethylamino)propyl)-10h-pyrido(3,2-b)(1,4)benzothiazine Hydrochloride
49. 10h-pyrido(3,2-b)(1,4)benzothiazine, 10-(2-dimethylamino-2-methylethyl)-, Hydrochloride
50. Isothipendyl Hydrochloride [mart.]
51. Cas-1225-60-1
52. Isothipendyl Hydrochloride [who-dd]
53. Ft-0700699
54. Isothipendyl Hydrochloride, (+/-)-
55. D01143
56. Q27271733
57. 10h-pyrido[3,4]benzothiazine, 10-(2-dimethylamino-2-methylethyl)-, Hydrochloride
58. 10h-pyrido[3,4]benzothiazine, 10-[2-(dimethylamino)propyl]-, Monohydrochloride
59. 10h-pyrido[3,4]benzothiazine-10-ethanamine, N,n,2-trimethyl-, Monohydrochloride
60. N,n,alpha-trimethyl-10h-pyrido[3,2-b][1,4]benzothiazine-10-ethanamine Hydrochloride
| Molecular Weight | 321.9 g/mol |
|---|---|
| Molecular Formula | C16H20ClN3S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 321.1066465 g/mol |
| Monoisotopic Mass | 321.1066465 g/mol |
| Topological Polar Surface Area | 44.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 323 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)