
1. Dynacirc
2. Isradipine, (+-)-isomer
3. Isradipine, (r)-isomer
4. Isradipine, (s)-isomer
5. Lomir
6. Pn 200-110
7. Pn 205 033
8. Pn 205 034
9. Pn 205-033
10. Pn 205-034
11. Pn 205033
12. Pn 205034
13. Pn-200-110
14. Pn-205-033
15. Pn-205-034
16. Pn205033
17. Pn205034
1. 75695-93-1
2. Dynacirc
3. Isradipin
4. Lomir
5. Isrodipine
6. Prescal
7. Esradin
8. Dynacirc Cr
9. Clivoten
10. Pn 200-110
11. Dynacrine
12. Isradipinum [latin]
13. Isradipino [spanish]
14. Pn-200-110
15. Rebriden
16. Dynacire
17. Dynacire Cr
18. (+/-)-isradipine
19. Isradipine (dynacirc)
20. Pn 200
21. C19h21n3o5
22. (+-)-isradipine
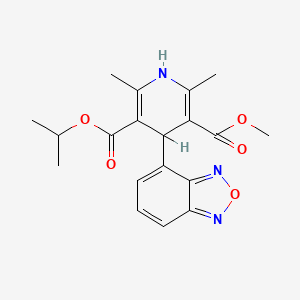
23. 3-isopropyl 5-methyl 4-(benzo[c][1,2,5]oxadiazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
24. 3-o-methyl 5-o-propan-2-yl 4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
25. 3,5-pyridinedicarboxylic Acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester
26. Nsc-759892
27. Yo1uk1s598
28. 131970-21-3
29. 3,5-pyridinedicarboxylic Acid, 4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester
30. Isradipine D3
31. Isradipinum
32. Isradipino
33. Dsstox_cid_3179
34. Dsstox_rid_76907
35. Dsstox_gsid_23179
36. Icaz
37. 4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinecarboxylic Acid Methyl 1-methylethyl Ester
38. Smr000466320
39. Smr002529690
40. Dynacirc (tn)
41. Isradipine (usp/inn)
42. Sr-01000597526
43. Mfcd00153820
44. Unii-yo1uk1s598
45. Isradipine [usan:usp:inn:ban]
46. Ncgc00016931-01
47. (-)-isradipine
48. (plusmn)-isradipine
49. Cas-75695-93-1
50. Pn200-110
51. Isopropyl Methyl (+-)-4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
52. Spectrum_000218
53. Isradipine [mi]
54. Pn-200110
55. Isradipine [inn]
56. Prestwick0_001021
57. Prestwick1_001021
58. Prestwick2_001021
59. Prestwick3_001021
60. (.+/-.)-isradipine
61. Isradipine [usan]
62. Isradipine [vandf]
63. Isradipine [mart.]
64. Chembl1648
65. Isradipine [usp-rs]
66. Isradipine [who-dd]
67. Schembl34555
68. Bspbio_001201
69. Kbioss_000698
70. 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid Methyl 1-methylethyl Ester
71. Mls000759425
72. Mls001424076
73. Mls002154106
74. Mls003915633
75. Mls006010138
76. O5-methyl O3-propan-2-yl 4-(2,1,3-benzoxadiazol-7-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
77. Spbio_003062
78. Bpbio1_001323
79. Chebi:6073
80. Chembl315436
81. Gtpl4488
82. Chembl3211306
83. Dtxsid4023179
84. Isradipine [orange Book]
85. Kbio2_000698
86. Kbio2_003266
87. Kbio2_005834
88. Isradipine [ep Monograph]
89. Hms1571m03
90. Hms2051j12
91. Hms2098m03
92. Hms2235o12
93. Hms3268d21
94. Hms3393j12
95. Hms3413a15
96. Hms3655c17
97. Hms3677a15
98. Hms3715m03
99. Hms3884e06
100. Isradipine [usp Monograph]
101. Pharmakon1600-02300234
102. (non-isotopelabelled)isradipine-d7
103. Act02697
104. Bcp06719
105. Hy-b0233
106. Benzo[1,2,5]oxadiazol-4-aldehyde
107. Tox21_110690
108. Bdbm50436176
109. Nsc759892
110. S1662
111. Akos015895129
112. Isradipine, >=98% (hplc), Solid
113. Tox21_110690_1
114. Ac-8539
115. Ccg-101027
116. Ccg-213711
117. Db00270
118. Hs-0085
119. Nc00277
120. Nsc 759892
121. Isopropyl Methyl 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
122. Ncgc00025341-01
123. Ncgc00025341-02
124. Ncgc00025341-03
125. Ncgc00025341-06
126. 3,5-pyridinedicarboxylic Acid, 4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester (9ci)
127. 3,5-pyridinedicarboxylic Acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester, (+-)-
128. 4-(2,1,3-benzooxadiazol-4-yl)-2.6-dimethyl-1,4-dihydro-3-isopropyloxycarbonylpyridine-5-carboxylic Acid Methyl Ester
129. 4-(4-benzofurazanyl)-1,-4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid Methyl 1-methhylethyl Ester
130. 4-(4-benzofurazanyl)-1,4-dihydro-2,-6-dimethyl-3,5-pyridinedicarboxylic Acid Methyl 1-methylethyl Ester
131. Isopropyl 4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-5-methoxycarbonyl-2,6-dimethyl-3-pyridinecarboxylate
132. Methyl 1-methylethyl 4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
133. Db-055981
134. Ab00514007
135. Ft-0627542
136. I0876
137. Sw220017-1
138. D00349
139. Ab00514007_02
140. Ab00514007_03
141. 695i931
142. Q414873
143. J-513454
144. Sr-01000597526-1
145. Sr-01000597526-2
146. Sr-01000597526-8
147. Brd-a90799790-001-03-3
148. Brd-a90799790-001-10-8
149. Isradipine, European Pharmacopoeia (ep) Reference Standard
150. Isradipine, United States Pharmacopeia (usp) Reference Standard
151. Isopropyl Methyl 4-(2,1,3-benzoxadiazol-4-yl)-1,4-dihydro-2,6-dimet
152. 3,5-pyridinedicarboxylic Acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester, (+/-)-
153. 3,5-pyridinedicarboxylic Acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, Methyl 1-methylethyl Ester, (.+/-.)-
154. 3-isopropyl 5-methyl 4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate #
155. 3-methyl 5-propan-2-yl 4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
156. 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid Isopropyl Methyl Ester
157. Isopropyl Methyl (+/-)-4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
158. Isopropyl Methyl (.+/-.)-4-(4-benzfurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
159. Isopropyl Methyl (.+/-.)-4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
| Molecular Weight | 371.4 g/mol |
|---|---|
| Molecular Formula | C19H21N3O5 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 371.14812078 g/mol |
| Monoisotopic Mass | 371.14812078 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 685 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Isradipine |
| PubMed Health | Isradipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Isradipine is a calcium antagonist available for oral administration in capsules containing 2.5 mg or 5 mg. The structural formula of isradipine is:Chemically, isradipine is 3,5-Pyridinedicarboxylic acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl... |
| Active Ingredient | Isradipine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg |
| Market Status | Prescription |
| Company | Watson Labs; Mikah Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Isradipine |
| PubMed Health | Isradipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Isradipine is a calcium antagonist available for oral administration in capsules containing 2.5 mg or 5 mg. The structural formula of isradipine is:Chemically, isradipine is 3,5-Pyridinedicarboxylic acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl... |
| Active Ingredient | Isradipine |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 2.5mg; 5mg |
| Market Status | Prescription |
| Company | Watson Labs; Mikah Pharma |
For the management of mild to moderate essential hypertension. It may be used alone or concurrently with thiazide-type diuretics.
Isradipine decreases arterial smooth muscle contractility and subsequent vasoconstriction by inhibiting the influx of calcium ions through L-type calcium channels. Calcium ions entering the cell through these channels bind to calmodulin. Calcium-bound calmodulin then binds to and activates myosin light chain kinase (MLCK). Activated MLCK catalyzes the phosphorylation of the regulatory light chain subunit of myosin, a key step in muscle contraction. Signal amplification is achieved by calcium-induced calcium release from the sarcoplasmic reticulum through ryanodine receptors. Inhibition of the initial influx of calcium decreases the contractile activity of arterial smooth muscle cells and results in vasodilation. The vasodilatory effects of isradipine result in an overall decrease in blood pressure.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA03 - Isradipine
Absorption
Isradipine is 90%-95% absorbed and is subject to extensive first-pass metabolism, resulting in a bioavailability of about 15%-24%.
Route of Elimination
Approximately 60% to 65% of an administered dose is excreted in the urine and 25% to 30% in the feces.
Hepatic. Completely metabolized prior to excretion and no unchanged drug is detected in the urine.
8 hours
Isradipine belongs to the dihydropyridine (DHP) class of calcium channel blockers (CCBs), the most widely used class of CCBs. There are at least five different types of calcium channels in Homo sapiens: L-, N-, P/Q-, R- and T-type. CCBs target L-type calcium channels, the major channel in muscle cells that mediates contraction. Similar to other DHP CCBs, isradipine binds directly to inactive calcium channels stabilizing their inactive conformation. Since arterial smooth muscle depolarizations are longer in duration than cardiac muscle depolarizations, inactive channels are more prevalent in smooth muscle cells. Alternative splicing of the alpha-1 subunit of the channel gives isradipine additional arterial selectivity. At therapeutic sub-toxic concentrations, isradipine has little effect on cardiac myocytes and conduction cells.