


1. Gs 9137
2. Gs-9137
3. Gs9137
4. Jtk 303
5. Jtk-303
6. Jtk303
7. Vitekta
1. 697761-98-1
2. Gs-9137
3. Jtk-303
4. Vitekta
5. Gs 9137
6. Elvitegravir (gs-9137)
7. Evg
8. Unii-4gdq854u53
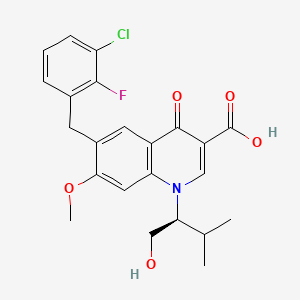
9. (s)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-methylbutan-2-yl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
10. 6-(3-chloro-2-fluorobenzyl)-1-[1(s)-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
11. Chebi:72289
12. 6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2s)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic Acid
13. Chembl204656
14. 4gdq854u53
15. D06677
16. 6-(3-chloro-2-fluorobenzyl)-1-((2s)-1-hydroxy-3-methylbutan-2-yl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
17. 6-(3-chloro-2-fluorobenzyl)-1-[(2s)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
18. Jtk 303
19. 3-quinolinecarboxylic Acid, 6-((3-chloro-2-fluorophenyl)methyl)-1,4-dihydro-1-((1s)-1-(hydroxymethyl)-2-methylpropyl)-7-methoxy-4-oxo-
20. 6-(3-chloro-2-fluorobenzyl)-1-[(1s)-1-(hydroxymethyl)-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
21. Jtk303
22. Elvitegravir [usan]
23. Gs9137
24. Elvitegravir [usan:inn]
25. Elvitegravirum
26. 6-(3-chloro-2-fluorobenzyl)-1-((1s)-1-(hydroxymethyl)-2-methylpropyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
27. 6-[(3-chloro-2-fluoro-phenyl)methyl]-1-[(1s)-1-(hydroxymethyl)-2-methyl-propyl]-7-methoxy-4-oxo-quinoline-3-carboxylic Acid
28. Elv
29. Vitekta (tn)
30. Elvitegravir [mi]
31. Elvitegravir (jan/usan)
32. Elvitegravir [inn]
33. Elvitegravir [jan]
34. Elvitegravir (jtk-303)
35. Elvitegravir [vandf]
36. Elvitegravir [mart.]
37. Mls006011136
38. Elvitegravir [who-dd]
39. Schembl726252
40. Gtpl11574
41. Bcpp000242
42. Dtxsid101021650
43. Elvitegravir [orange Book]
44. Ex-a1542
45. Bdbm50183273
46. Mfcd11846134
47. S2001
48. Vitekta Component Elvitegravir
49. Zinc13682481
50. Elvitegravir; Gs9137; Jtk 303
51. Stribild Component Elvitegravir
52. Akos025396642
53. Bcp9000642
54. Ccg-269208
55. Compound 2 [pmid: 18281931]
56. Cs-0439
57. Db09101
58. Elvitegravir (gs-9137, Jtk-303)
59. Elvitegravir Component Of Genvoya
60. Elvitegravir Component Of Vitekta
61. Elvitegravir Component Of Stribild
62. Ncgc00346565-01
63. Ncgc00346565-04
64. Ncgc00346565-08
65. Ac-29947
66. As-16986
67. Hy-14740
68. Smr004702914
69. Elvitegravir 100 Microg/ml In Acetonitrile
70. A4070
71. Sw219721-1
72. Ec-000.2332
73. Ab01274749-01
74. Ab01274749_02
75. J-518006
76. Q2740966
77. Brd-k54472332-001-01-8
78. 3-quinolinecarboxylic Acid, 6-[(3-chloro-2-fluorophenyl)methyl]-1,4-dihydro-1-[(1s)-1-isopropyl-2-hydroxyethyl]-7-methoxy-4-oxo-
79. 6-(3-chloro-2-fluorobenzyl)-1-[(s)-1-hydroxymethyl-2-methylpropyl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid
| Molecular Weight | 447.9 g/mol |
|---|---|
| Molecular Formula | C23H23ClFNO5 |
| XLogP3 | 5.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 447.1248787 g/mol |
| Monoisotopic Mass | 447.1248787 g/mol |
| Topological Polar Surface Area | 87.1 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 702 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Elvitegravir in combination with an HIV protease inhibitor coadministered with ritonavir and with other antiretroviral drug(s) is indicated for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults.
FDA Label
Vitekta co-administered with a ritonavir-boosted protease inhibitor and with other antiretroviral agents, is indicated for the treatment of human-immunodeficiency-virus-1 (HIV-1) infection in adults who are infected with HIV-1 without known mutations associated with resistance to elvitegravir.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Anti-Retroviral Agents
Agents used to treat RETROVIRIDAE INFECTIONS. (See all compounds classified as Anti-Retroviral Agents.)
Integrase Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of integrase. (See all compounds classified as Integrase Inhibitors.)
J05AX11
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AJ - Integrase inhibitors
J05AJ02 - Elvitegravir
Absorption
Following oral administration of elvitegravir and ritonavir with food, in HIV-1 infected subjects, peak elvitegravir plasma concentrations were observed approximately 4 hours post-dose.
Route of Elimination
Following oral administration of [14C]elvitegravir/ritonavir, 94.8% of the dose was recovered in feces, while 6.7% was recovered in urine as metabolites.
Elvitegravir undergoes primarily oxidative metabolism via CYP3A, and is secondarily glucuronidated via UGT1A1/3 enzymes. Metabolites are found in the plasma at very low concentrations, displayed considerably lower anti-HIV activity, and did not contribute to the overall antiviral activity of elvitegravir.
The median terminal plasma half-life following administration of elvitegravir and ritonavir was approximately 8.7 hours.
Elvitegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Integrase is an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II.