
1. 19,583 Rp
2. 2-(3-benzoylphenyl)propionic Acid
3. Alrheumat
4. Alrheumum
5. Benzoylhydratropic Acid
6. Orudis
7. Profenid
8. Rp 19583
9. Rp, 19,583
10. Rp-19583
11. Rp19583
1. 22071-15-4
2. 2-(3-benzoylphenyl)propanoic Acid
3. Orudis
4. 2-(3-benzoylphenyl)propionic Acid
5. Actron
6. Capisten
7. Oruvail
8. M-benzoylhydratropic Acid
9. Alrheumat
10. Ketoprofene
11. Profenid
12. Aneol
13. 3-benzoylhydratropic Acid
14. Alrheumun
15. Epatec
16. Iso-k
17. Orudis (tn)
18. Rp-19583
19. Ketoprofeno
20. Ketoprophene
21. 2-[3-(phenylcarbonyl)phenyl]propanoic Acid
22. Ketorin
23. Sector
24. (rs)-ketoprofen
25. 2-(m-benzoylphenyl)propionic Acid
26. Actron Ketoprofen
27. Racemic Ketoprofen
28. Benzeneacetic Acid, 3-benzoyl-.alpha.-methyl-
29. 3-benzoyl-alpha-methylbenzeneacetic Acid
30. Ru 4733
31. Ketoprofen (actron)
32. Chebi:6128
33. Chembl571
34. Nsc-758144
35. Propionic Acid, 2-(3-benzoylphenyl)-
36. (s)-ketoprofen;dexketoprofen
37. 19583 Rp
38. Hydratropic Acid, M-benzoyl-
39. L'acide (benzoyl-3-phenyl)-2-propionique
40. Mls000079024
41. Idea-033
42. R.p. 19,583
43. 90y4qc304k
44. Orudis Kt
45. 1189508-77-7
46. 19583rp
47. Ru-4733
48. Kefenid
49. Ketopron
50. Menamin
51. Meprofen
52. Orugesic
53. Oscorel
54. Smr000040181
55. Fastum
56. Ketoprofen 100 Microg/ml In Acetonitrile
57. Lertus
58. Toprec
59. Toprek
60. Dexal
61. Dsstox_cid_771
62. R.p. 19583
63. Racemic-ketoprofen
64. Dsstox_rid_75783
65. Dsstox_gsid_20771
66. Ketoprofenum
67. Rp 19583
68. Ketoprofene [inn-french]
69. Ketoprofenum [inn-latin]
70. Ketoprofeno [inn-spanish]
71. Ketoprofen (+-)
72. (+-)-m-benzoylhydratropic Acid
73. Benzeneacetic Acid, 3-benzoyl-alpha-methyl-
74. Sr-01000075949
75. Rac Ketoprofen
76. Ccris 4508
77. (+-)-3-benzoyl-alpha-methylbenzeneacetic Acid
78. (+/-)-m-benzoylhydratropic Acid
79. (+) Ketoprofen
80. Ncgc00016757-01
81. Ketoprofen ,(s)
82. Acide (benzoyl-3-phenyl)-2-propionique [french]
83. Prestwick_617
84. Einecs 244-759-8
85. 2-[3-(benzoyl)phenyl]propanoic Acid
86. Cas-22071-15-4
87. Mfcd00055790
88. Nexcede
89. Ketoprofen-13c-d3
90. 2-(3-benzoylphenyl)-propionic Acid
91. Hydratropic Acid, M-benzoyl-, (+-)-
92. Acide (benzoyl-3-phenyl)-2-propionique
93. Spectrum_001309
94. Ketoprofen [mi]
95. Opera_id_509
96. Ketoprofen [inn]
97. Ketoprofen [jan]
98. Prestwick0_000219
99. Prestwick1_000219
100. Prestwick2_000219
101. Prestwick3_000219
102. Spectrum2_000956
103. Spectrum3_001479
104. Spectrum4_000028
105. Spectrum5_001254
106. Ketoprofen [usan]
107. M-benzoyl-hydratropic Acid
108. Ketoprofen [vandf]
109. Epitope Id:131783
110. K 1751
111. Ketoprofen [mart.]
112. Schembl2896
113. Ketoprofen [who-dd]
114. (+/-)-ketoprofen
115. Lopac0_000686
116. Oprea1_117113
117. Bspbio_000237
118. Bspbio_003037
119. Kbiogr_000435
120. Kbioss_001789
121. 22161-86-0
122. Mls000028446
123. Mls001201752
124. Mls001306444
125. Mls002548889
126. Mls006011967
127. Bidd:gt0443
128. Divk1c_000598
129. Spectrum1501215
130. Unii-90y4qc304k
131. Spbio_000952
132. Spbio_002158
133. Benzeneacetic Acid, 3-benzoyl-alpha-methyl-, (+-)-
134. Bpbio1_000261
135. Gtpl4795
136. Ketoprofen (jp17/usp/inn)
137. Ketoprofen [green Book]
138. Ketoprofen, >=98% (tlc)
139. Dtxsid6020771
140. Ketoprofen [ep Impurity]
141. Ketoprofen [orange Book]
142. Dkywvdodhfezim-uhfffaoysa-
143. Hms501n20
144. Kbio1_000598
145. Kbio2_001789
146. Kbio2_004357
147. Kbio2_006925
148. Kbio3_002537
149. 2-(3-benzoylphenyl)propanoicacid
150. Ketoprofen [ep Monograph]
151. Ketoprofen [usp Impurity]
152. Ninds_000598
153. Hms1568l19
154. Hms1921b12
155. Hms2089b16
156. Hms2092l19
157. Hms2095l19
158. Hms2234h16
159. Hms3259i05
160. Hms3262i13
161. Hms3372m08
162. Hms3373g09
163. Hms3649n10
164. Hms3655c15
165. Hms3712l19
166. Hms3884k04
167. Ketoprofen [usp Monograph]
168. Pharmakon1600-01501215
169. Bcp23428
170. Hy-b0227
171. 2-(3'-benzoylphenyl)propionic Acid
172. 2-(3-benzoylphenyl) Propionic Acid
173. Alpha(3-benzoylphenyl)propionic Acid
174. Tox21_110594
175. Tox21_200847
176. Tox21_500686
177. (.+/-.)-m-benzoylhydratropic Acid
178. 2-(3-benzoylphenyl) Propionoic Acid
179. Alpha-(m-benzoylphenyl)propionic Acid
180. Bdbm50022271
181. Ccg-39685
182. Nsc758144
183. S1645
184. Stl450995
185. (r)-(-)-ketoprofen-[13c,d3]
186. 2-(3-benzoylphenyl)propanoic Acid #
187. Alpha-(3-benzoylphenyl)propionic Acid
188. Akos007930512
189. Alpha-(m-benzoylphenyl) Propionic Acid
190. Ketoprofen 100 Microg/ml In Methanol
191. Tox21_110594_1
192. Ac-1486
193. Bcp9000810
194. Db01009
195. Ketoprofen [usan:usp:inn:ban:jan]
196. Ks-5031
197. Lp00686
198. Nc00459
199. Nsc 758144
200. Sdccgsbi-0050664.p004
201. Idi1_000598
202. (rs)-2-(3-benzoylphenyl)propanoic Acid
203. Ncgc00015578-02
204. Ncgc00015578-03
205. Ncgc00015578-04
206. Ncgc00015578-05
207. Ncgc00015578-07
208. Ncgc00015578-08
209. Ncgc00015578-10
210. Ncgc00015578-12
211. Ncgc00015578-23
212. Ncgc00094043-01
213. Ncgc00094043-02
214. Ncgc00094043-03
215. Ncgc00094043-04
216. Ncgc00258401-01
217. Ncgc00261371-01
218. Bk166172
219. ((c)i)-ketoprofen-d4(propionic-d4 Acid)
220. 3-benzoyl-.alpha.-methylbenzeneacetic Acid
221. Bcp0726000302
222. Sbi-0050664.p003
223. (+/-)-2-(3-benzoylphenyl)propionic Acid
224. L''acide (benzoyl-3-phenyl)-2-propionique
225. Unm-0000306100
226. Ab00052249
227. Am20060549
228. Eu-0100686
229. Ft-0602834
230. Ft-0670646
231. Ft-0670647
232. K0038
233. Ketoprofen, Meets Usp Testing Specifications
234. Orudis, Profenid, Dexal, Keduril, Ketofen,
235. Sw196784-3
236. Bim-0050664.0001
237. C01716
238. D00132
239. D78110
240. Ketoprofen, Vetranal(tm), Analytical Standard
241. Ab00052249-17
242. Ab00052249-19
243. Ab00052249-20
244. Ab00052249_21
245. Ab00052249_22
246. 071k154
247. A815896
248. Q409192
249. Q-201268
250. Sr-01000075949-1
251. Sr-01000075949-6
252. Sr-01000075949-9
253. (.+/-.)-3-benzoyl-.alpha.-methylbenzeneacetic Acid
254. Brd-a97739905-001-05-9
255. Brd-a97739905-001-15-8
256. Sr-01000075949-18
257. F2173-0960
258. Z1695709452
259. (+/-)-3-benzoyl-.alpha.-methylbenzeneacetic Acid
260. Ketoprofen, British Pharmacopoeia (bp) Reference Standard
261. Ketoprofen, European Pharmacopoeia (ep) Reference Standard
262. Ketoprofen, United States Pharmacopeia (usp) Reference Standard
263. N-fmoc-3-amino-4-(4-tert-butoxy-phenyl)-butyricacid
264. Ketoprofen, Pharmaceutical Secondary Standard; Certified Reference Material
265. 154907-35-4
| Molecular Weight | 254.28 g/mol |
|---|---|
| Molecular Formula | C16H14O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 254.094294304 g/mol |
| Monoisotopic Mass | 254.094294304 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Ketoprofen |
| PubMed Health | Ketoprofen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
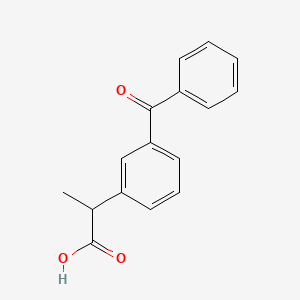
| Drug Label | Ketoprofen is a non-steroidal anti-inflammatory drug. The chemical name for ketoprofen is 2-(3-benzoylphenyl)-propionic acid with the following structural formula:C16H14O3M.W. 254.29It has a pKa of 5.94 in methanol: water (3:1) an... |
| Active Ingredient | Ketoprofen |
| Dosage Form | Capsule; Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg; 150mg; 75mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Teva; Dorado Pharma; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Ketoprofen |
| PubMed Health | Ketoprofen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Ketoprofen is a non-steroidal anti-inflammatory drug. The chemical name for ketoprofen is 2-(3-benzoylphenyl)-propionic acid with the following structural formula:C16H14O3M.W. 254.29It has a pKa of 5.94 in methanol: water (3:1) an... |
| Active Ingredient | Ketoprofen |
| Dosage Form | Capsule; Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 25mg; 150mg; 75mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Teva; Dorado Pharma; Mylan |
For symptomatic treatment of acute and chronic rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, primary dysmenorrhea and mild to moderate pain associated with musculotendinous trauma (sprains and strains), postoperative (including dental surgery) or postpartum pain.
FDA Label
Treatment of musculoskeletal and connective tissue pain
Ketoprofen is a nonsteroidal anti-inflammatory agent (NSAIA) with analgesic and antipyretic properties. Ketoprofen has pharmacologic actions similar to those of other prototypical NSAIDs, which inhibit prostaglandin synthesis. Ketoprofen is used to treat rheumatoid arthritis, osteoarthritis, dysmenorrhea, and alleviate moderate pain.
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M02AA10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE03 - Ketoprofen
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA10 - Ketoprofen
Absorption
Ketoprofen is rapidly and well-absorbed orally, with peak plasma levels occurring within 0.5 to 2 hours.
Route of Elimination
In a 24 hour period, approximately 80% of an administered dose of ketoprofen is excreted in the urine, primarily as the glucuronide metabolite.
Clearance
Oral-dose cl=6.9 +/- 0.8 L/h [Ketoprofen Immediate-release capsules (4 50 mg)]
Oral-dose cl=6.8 +/- 1.8 L/h [Ketoprofen Extended-release capsules (1 200 mg)]
0.08 L/kg/h
0.7 L/kg/h [alcoholic cirrhosis patients]
Rapidly and extensively metabolized in the liver, primarily via conjugation to glucuronic acid. No active metabolites have been identified.
Ketoprofen has known human metabolites that include Ketoprofen glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Conventional capsules: 1.1-4 hours
Extended release capsules: 5.4 hours due to delayed absorption (intrinsic clearance is same as conventional capsules)
The anti-inflammatory effects of ketoprofen are believed to be due to inhibition cylooxygenase-2 (COX-2), an enzyme involved in prostaglandin synthesis via the arachidonic acid pathway. This results in decreased levels of prostaglandins that mediate pain, fever and inflammation. Ketoprofen is a non-specific cyclooxygenase inhibitor and inhibition of COX-1 is thought to confer some of its side effects, such as GI upset and ulceration. Ketoprofen is thought to have anti-bradykinin activity, as well as lysosomal membrane-stabilizing action. Antipyretic effects may be due to action on the hypothalamus, resulting in an increased peripheral blood flow, vasodilation, and subsequent heat dissipation.