

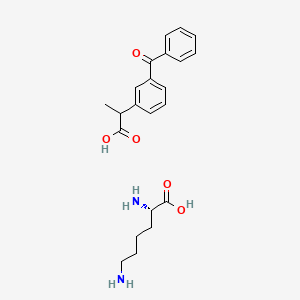

1. Artrosilene
2. Lysyl-ketoprofen
1. Ketoprofen Lysinate
2. 57469-78-0
3. Ketoprofen Lysine Salt
4. Lysyl-ketoprofen
5. Artrosilene
6. Ketoprofen L-lysinate
7. Ketoprofen (lysinate)
8. L-lysine, Mono(3-benzoyl-alpha-methylbenzeneacetate)
9. 5wd00e3d4c
10. (s)-2,6-diaminohexanoic Acid Compound With 2-(3-benzoylphenyl)propanoic Acid (1:1)
11. 2-(3-benzoylphenyl)propanoic Acid;(2s)-2,6-diaminohexanoic Acid
12. Unii-5wd00e3d4c
13. Artrosilene (tn)
14. Schembl4623586
15. Chembl4650345
16. Hy-b0227a
17. Ketoprofen Lysine [who-dd]
18. Ketoprofen Lysine Salt [mi]
19. Mfcd03701078
20. Akos025311486
21. As-17318
22. Cs-0137969
23. D08102
24. 469k780
25. Q27262959
26. L-lysine, 3-benzoyl-.alpha.-methylbenzeneacetate (1:1)
| Molecular Weight | 400.5 g/mol |
|---|---|
| Molecular Formula | C22H28N2O5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 400.19982200 g/mol |
| Monoisotopic Mass | 400.19982200 g/mol |
| Topological Polar Surface Area | 144 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 437 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
