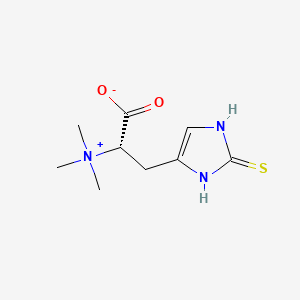

1. 2 Thiol L Histidine Betaine
2. 2-thiol-l-histidine-betaine
3. Thioneine
1. L-(+)-ergothioneine
2. 497-30-3
3. L-ergothioneine
4. Sympectothion
5. Ergothionine
6. L-thioneine
7. Thiolhistidine-betaine
8. Thioneine
9. (s)-3-(2-thioxo-2,3-dihydro-1h-imidazol-4-yl)-2-(trimethylammonio)propanoate
10. Bdz3dqm98w
11. 58511-63-0
12. L(+)-ergothioneine
13. Thiasine
14. (2s)-3-(2-sulfanylidene-1,3-dihydroimidazol-4-yl)-2-(trimethylazaniumyl)propanoate
15. (s)-(1-carboxy-2-(2-mercaptoimidazol-4-yl)ethyl)trimethylammonium Hydroxide
16. Unii-bdz3dqm98w
17. Erythrothioneine
18. L-ergothionine
19. Ergothioneine Thiol
20. Ergothioneine Thione
21. Nsc 7175
22. Thiolhistidinebetaine
23. Einecs 207-843-5
24. Mfcd00167474
25. Ergothioneine L-(+)
26. Ergothioneine Thione Form
27. Ai3-23492
28. Ergothioneine [mi]
29. Ergothioneine (thione Form)
30. Ergothioneine [inci]
31. Schembl188140
32. Chebi:4828
33. Schembl9985141
34. Chebi:82707
35. Dtxsid901020082
36. Hms2089d04
37. 2-mercaptohistidine Trimethylbetaine
38. Hy-n1914
39. Ccg-207908
40. 1h-imidazole-4-ethanaminium, .alpha.-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-, Inner Salt, (.alpha.s)-
41. 1h-imidazole-4-ethanaminium, Alpha-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-, Inner Salt, (s)-
42. Cs-0018211
43. D83035
44. Ab01275464-01
45. A930109
46. Ergothionine;l-(+)-ergothioneine;erythrothioneine
47. Q614788
48. (2s)-3-(2-mercapto-1h-imidazol-5-yl)-2-(trimethylammonio)propanoate
49. (2s)-3-(2-mercapto-1h-imidazol-5-yl)-2-(trimethylazaniumyl)propanoate
50. 3-(2-sulfanylidene-1,3-dihydroimidazol-4-yl)-2-trimethylammonio-propanoate
51. Nalpha,nalpha,nalpha-trimethyl-2-sulfanylidene-2,3-dihydro-l-histidine
52. (2s)-3-(2-sulfanylidene-2,3-dihydro-1h-imidazol-4-yl)-2-(trimethylazaniumyl)propanoate
53. (2s)-3-(2-thioxo-2,3-dihydro-1h-imidazol-4-yl)-2-(trimethylazaniumyl)propanoate
54. (.alpha.s)-.alpha.-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-1h-imidazole-4-ethanaminium Inner Salt
55. (alphas)-alpha-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-1h-imidazole-4-ethanaminium Inner Salt
56. 1h-imidazole-4-ethanaminium, Alpha-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-, Hydroxide, Inner Salt, (s)-
57. 1h-imidazole-4-ethanaminium, Alpha-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-, Inner Salt, (alphas)-
58. 1h-imidazole-4-ethaniminium, Alpha-carboxy-2,3-dihydro-n,n,n-trimethyl-2-thioxo-, Hydroxide, Inner Salt, (s)-
59. Ammonium, (1-carboxy-2-(2-mercaptoimidazol-4-yl)ethyl)trimethyl-, Hydroxide, Inner Salt, L-(+)-
| Molecular Weight | 229.30 g/mol |
|---|---|
| Molecular Formula | C9H15N3O2S |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 96.3 |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 314 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)