


1. Frg 8813
2. Frg-8813
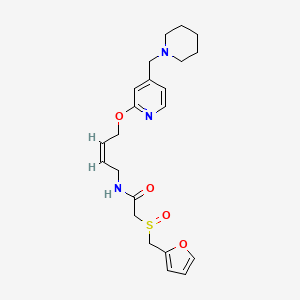
3. N-(4-(4-piperidinylmethyl)pyridyl-2-oxy)-(z)-butenyl-2-(furfurylsulfinyl)acetamide
4. N-(4-(4-piperidinylmethyl)pyridyl-2-oxy)butenyl-2-(furfurylsulfinyl)acetamide
1. 118288-08-7
2. 206449-93-6
3. Frg-8813
4. Lafutidine [inn]
5. (z)-lafutidine
6. Lafutidine [jan]
7. Lafutidine Free Base
8. Rac Lafutidine
9. Lafutidine [mart.]
10. Lafutidine [who-dd]
11. Lafutidine, (+/-)-
12. (z)-2-((furan-2-ylmethyl)sulfinyl)-n-(4-((4-(piperidin-1-ylmethyl)pyridin-2-yl)oxy)but-2-en-1-yl)acetamide
13. 49s4o7adlc
14. 118288-08-7 (free Base)
15. 2-(furan-2-ylmethylsulfinyl)-n-[(z)-4-[4-(piperidin-1-ylmethyl)pyridin-2-yl]oxybut-2-enyl]acetamide
16. Acetamide, 2-[(2-furanylmethyl)sulfinyl]-n-[(2z)-4-[[4-(1-piperidinylmethyl)-2-pyridinyl]oxy]-2-buten-1-yl]-
17. Unii-49s4o7adlc
18. Dsstox_cid_26434
19. Dsstox_rid_81611
20. Dsstox_gsid_46434
21. Protecadin
22. (+/-)-2-(furfurylsulfinyl)-n-((z)-4-((4-(piperidinomethyl)-2-pyridyl)oxy)-2-butenyl) Acetamide
23. Cas-118288-08-7
24. Frg 8813
25. Laftidine
26. Stogar
27. Protecadin (tn)
28. Ncgc00164550-01
29. N-(4-(4-piperidinylmethyl)pyridyl-2-oxy)butenyl-2-(furfurylsulfinyl)acetamide
30. Starbld0049801
31. Lafutidine (jp17/inn)
32. Mls006011259
33. Schembl362540
34. Chembl1742461
35. Dtxsid0046434
36. Lafutidine, >=98% (hplc)
37. Chebi:31759
38. Hms3884d03
39. Act06288
40. Hy-b0160
41. Tox21_112179
42. Mfcd21607521
43. S2065
44. Akos005146275
45. Tox21_112179_1
46. Ccg-269024
47. Cs-1992
48. Db12770
49. (+-)-2-(furfurylsulfinyl)-n-(4-(4-(piperidinomethyl)-2-pyridyl)oxy-(z)-2-butenyl)acetamide
50. Ncgc00263530-01
51. (+-)-2-(furfurylsulfinyl)-n-((z)-4-((4-(piperidinomethyl)-2-pyridyl)oxy)-2-butenyl) Acetamide
52. Ac-23354
53. Acetamide, 2-((2-furanylmethyl)sulfinyl)-n-(4-((4-(1-piperidinylmethyl)-2-pyridinyl)oxy)-2-butenyl)-, (z)-
54. Smr002529578
55. L0341
56. Sw219706-1
57. D01131
58. F77861
59. Ab01565815_02
60. 288l087
61. L001355
62. Q582556
63. J-521629
64. F0001-2391
| Molecular Weight | 431.6 g/mol |
|---|---|
| Molecular Formula | C22H29N3O4S |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 431.18787759 g/mol |
| Monoisotopic Mass | 431.18787759 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 569 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Histamine H2 Antagonists
Drugs that selectively bind to but do not activate histamine H2 receptors, thereby blocking the actions of histamine. Their clinically most important action is the inhibition of acid secretion in the treatment of gastrointestinal ulcers. Smooth muscle may also be affected. Some drugs in this class have strong effects in the central nervous system, but these actions are not well understood. (See all compounds classified as Histamine H2 Antagonists.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02B - Drugs for peptic ulcer and gastro-oesophageal reflux disease (gord)
A02BA - H2-receptor antagonists
A02BA08 - Lafutidine