
1. Fosrenol
2. Lantharenol
1. Foznol
2. 587-26-8
3. Lanthanum Sesquicarbonate
4. Phosbloc
5. Carbonic Acid, Lanthanum(3+) Salt (3:2)
6. Lanthanum(3+);tricarbonate
7. Lanthanum(3+) Carbonate
8. Lanthanum (iii) Carbonate
9. Lanthanum Carbonate (2:3)
10. Lanthanum Carbonate Anhydrous
11. 0m78eu4v9h
12. Dilanthanum Tricarbonate
13. Carbonic Acid,lanthanum(3+) Salt (3:2), Octahydrate (8ci,9ci)
14. Lanthanum(3 ) Carbonate
15. Einecs 209-599-5
16. Lanthanum(3+) Tricarbonate
17. Unii-0m78eu4v9h
18. Hsdb 7758
19. Carbonato De Lantano
20. Carbonate De Lanthane
21. Carbonato De Lantanio
22. Carbonato Di Lantanio
23. Lanthanum(iii) Carbonate
24. Lanthanum Carbonate Powder
25. Ec 209-599-5
26. Lanthanum(3+) Salt (3:2)
27. Lanthanum Carbonate [mi]
28. Dtxsid30890512
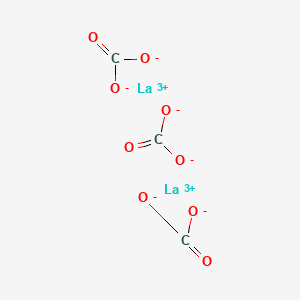
29. Lanthanum Carbonate (la2(co3)3)
30. Lanthanum Carbonate [who-dd]
31. Db06792
32. Lanthanum(3 ) Carbonate [hsdb]
33. Q421317
34. Carbonic Acid,lanthanum(3+) Salt (3:2), Hydrate (9ci)
| Molecular Weight | 457.84 g/mol |
|---|---|
| Molecular Formula | C3La2O9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 0 |
| Exact Mass | 457.76696 g/mol |
| Monoisotopic Mass | 457.76696 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
Lanthanum; Kidney Failure, Chronic
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Lanthanum carbonate is indicated to reduce serum phosphate in patients with end stage renal disease. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
Adverse effects reported in 5% or more of patients receiving lanthanum carbonate and more frequently than with placebo include nausea, vomiting, dialysis graft occlusion, and abdominal pain.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2755
Patients with acute peptic ulcer, ulcerative colitis, Crohn's disease or bowel obstruction were not included in lanthanum carbonate clinical studies. Caution should be used in patients with these conditions.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
While growth abnormalities were not identified in long-term animal studies, lanthanum was deposited into developing bone including growth plate. The consequences of such deposition in developing bone in pediatric patients are unknown. Therefore, the use of lanthanum carbonate in this population is not recommended.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
/Patients taking lanthanum carbonate should be advised of the/ importance of taking lanthanum carbonate with or immediately after meals.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2755
For more Drug Warnings (Complete) data for Lanthanum Carbonate (9 total), please visit the HSDB record page.
Used to reduce serum phosphate in patients with end stage renal disease (ESRD).
FDA Label
In vitro studies have shown that lanthanum binds phosphate in the physiologically relevant pH range of 3 to 7. In simulated gastric fluid, lanthanum binds approximately 97% of the available phosphate at pH 3-5 and 67% at pH 7, when lanthanum is present in a two-fold molar excess to phosphate. Bile acids have not been shown to affect the phosphate binding affinity of lanthanum. In order to bind dietary phosphate, lanthanum carbonate must be administered with or immediately after meals.
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AE - Drugs for treatment of hyperkalemia and hyperphosphatemia
V03AE03 - Lanthanum carbonate
Absorption
Bioavailability very low (<0.002%) following single or multiple dose oral administration.
Route of Elimination
No information is available regarding the mass balance of lanthanum in humans after oral administration. In rats and dogs, the mean recovery of lanthanum after an oral dose was about 99% and 94%, respectively, and was essentially all from feces. Biliary excretion is the predominant route of elimination for circulating lanthanum in rats.
Clearance
In healthy volunteers administered intravenous lanthanum as the soluble chloride salt (120 g), renal clearance was less than 2% of total plasma clearance.
Lanthanum is minimally absorbed from the GI tract following oral administration; bioavailability is less than 0.002%. In patients receiving therapeutic dosages of lanthanum for up to 2 years, mean plasma concentrations of the drug remained low (ie, 1.1 ng/mL or less).
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2755
Following single or multiple dose oral administration of lanthanum carbonate to healthy subjects, the concentration of lanthanum in plasma was very low. Following oral administration in ESRD patients, the mean lanthanum Cmax was 1.0 ng/mL. During long-term administration (52 weeks) in ESRD patients, the mean lanthanum concentration in plasma was approximately 0.6 ng/mL. There was minimal increase in plasma lanthanum concentrations with increasing doses within the therapeutic dose range. The effect of food on the bioavailability of lanthanum carbonate has not been evaluated, but the timing of food intake relative to lanthanum administration (during and 30 minutes after food intake) has a negligible effect on the systemic level of lanthanum
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
In vitro, lanthanum is highly bound (>99%) to human plasma proteins, including human serum albumin, alpha1-acid glycoprotein, and transferrin. Binding to erythrocytes in vivo is negligible in rats.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
In studies in mice, rats and dogs, lanthanum concentrations in many tissues increased over time and were several orders of magnitude higher than plasma concentrations (particularly in the GI tract, bone and liver). Steady state tissue concentrations in bone and liver were achieved in dogs between 4 and 26 weeks. Relatively high levels of lanthanum remained in these tissues for longer than 6 months after cessation of dosing in dogs. There is no evidence from animal studies that lanthanum crosses the blood-brain barrier.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
For more Absorption, Distribution and Excretion (Complete) data for Lanthanum Carbonate (13 total), please visit the HSDB record page.
Lanthanum is not metabolized.
Lanthanum is not metabolized and is not a substrate of CYP450. In vitro metabolic inhibition studies showed that lanthanum at concentrations of 10 and 40 ug/mL does not have relevant inhibitory effects on any of the CYP450 isoenzymes tested (1A2, 2C9/10, 2C19, 2D6, and 3A4/5).
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
Elimination half-life of 53 hours.
Lanthanum was cleared from plasma following discontinuation of therapy with an elimination half-life 53 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
... Estimates of elimination half-life from bone ranged from 2.0 to 3.6 years.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
Lanthanum carbonate is a phosphate binder that reduces absorption of phosphate by forming insoluble lanthanum phosphate complexes that pass through the gastrointestinal (GI) tract unabsorbed. Both serum phosphate and calcium phosphate product are reduced as a consequence of the reduced dietary phosphate absorption.
Lanthanum carbonate dissociates in the acid environment of the upper GI tract to release lanthanum ions that bind dietary phosphate released from food during digestion. Lanthanum carbonate inhibits absorption of phosphate by forming highly insoluble lanthanum phosphate complexes, consequently reducing both serum phosphate and calcium phosphate product.
US Natl Inst Health; DailyMed. Current Medication Information for Fosrenol (Lanthanum carbonate) (January 2006). Available from, as of June 17, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3132
The carbonate salt of lanthanum is practically insoluble in water but dissociates in the acidic environment of the upper GI tract to release trivalent lanthanum ions, which bind dietary phosphates released during digestion, thereby forming highly insoluble lanthanum phosphate complexes. Consequently, phosphate absorption, serum phosphorus concentrations, and serum calcium times phosphorus product (Ca X P) are reduced. Lanthanum ions have a high affinity for phosphate; in vitro studies indicate that when lanthanum is present at pH 3-5 (pH corresponding to that of gastric fluid) in twofold molar excess to phosphates, the drug binds about 97% of available phosphates.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2755