


1. Abr 215062
2. Abr-215062
3. Abr215062
1. 248281-84-7
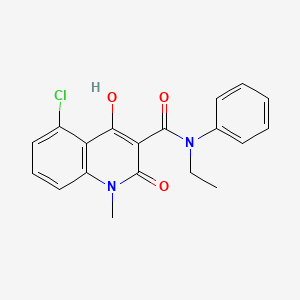
2. 5-chloro-n-ethyl-4-hydroxy-1-methyl-2-oxo-n-phenyl-1,2-dihydroquinoline-3-carboxamide
3. Abr-215062
4. Abr 215062
5. Laquinimod [inn]
6. Civentichem Cv-4057
7. Laquinimod (abr-215062)
8. Abr215062
9. Tv-5600 Free Acid
10. 908sy76s4g
11. Abr-215062 Free Acid
12. 5-chloro-n-ethyl-4-hydroxy-1-methyl-2-oxo-n-phenylquinoline-3-carboxamide
13. N-ethyl-n-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide
14. Mfcd08689859
15. 5-chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic Acid Ethyl-phenyl-amide
16. 3-quinolinecarboxamide, 5-chloro-n-ethyl-1,2-dihydro-4-hydroxy-1-methyl-2-oxo-n-phenyl-
17. Smr004701305
18. Unii-908sy76s4g
19. Laquinimod [mi]
20. Laquinimod [who-dd]
21. Laquinimod,abr-215062
22. Schembl39440
23. Schembl39441
24. Mls006010210
25. Mls006010286
26. Chembl66092
27. Gtpl7639
28. Abr-215062 (laquinimod)
29. Amy6804
30. Dtxsid30179536
31. Ex-a079
32. Laquinimod Pound Notabr215062
33. Chebi:134738
34. Hms3656n08
35. Bcp04521
36. Zinc5573462
37. S2787
38. Akos005146322
39. Zinc100004621
40. Bcp9000840
41. Ccg-268120
42. Cs-0839
43. Db06685
44. Pb32648
45. 5-chloro-n-et-4-hydroxy-1-methyl-2-oxo-n-ph-1,2-dihydroquinoline-3-carboxamide
46. N-ethyl-n-phenyl-5-chloro-1,2-dihydro-4-hydroxy-1-methyl-2-oxoquinoline-3-carboxamide
47. Ncgc00346701-01
48. Ncgc00346701-07
49. Bs-16743
50. Hy-13010
51. Laquinimod,5-chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic Acid Ethyl-phenyl-amide
52. Bcp0726000056
53. Ft-0698088
54. Sw220142-1
55. J-521632
56. Q3487584
57. 5-chloro-4-hydroxy-1-methyl-2-oxo-n-ethyl-n-phenyl-1,2-dihydroquinoline-3-carboxamide
58. N-ethyl-n-phenyl-5-chloro-1,2-dihydro-4-hydroxy-1-methyl-2-oxo-quinoline-3-carboxamide
| Molecular Weight | 356.8 g/mol |
|---|---|
| Molecular Formula | C19H17ClN2O3 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 356.0927701 g/mol |
| Monoisotopic Mass | 356.0927701 g/mol |
| Topological Polar Surface Area | 60.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 571 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in multiple sclerosis.
Treatment of multiple sclerosis
N07
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX10 - Laquinimod
Hepatic. Cytochrome P450 3A4 is the major enzyme responsible for the metabolism of laquinimod.
Laquinimod has known human metabolites that include 5-chloro-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide, 5-chloro-N-ethyl-4,6-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide, 5-chloro-N-ethyl-4,7-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide, 5-chloro-N-ethyl-4,8-dihydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide, 5-chloro-N-ethyl-4-hydroxy-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide, and 5-chloro-N-ethyl-4-hydroxy-N-(4-hydroxyphenyl)-1-methyl-2-oxo-1,2-dihydroquinoline-3-carboxamide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560