


1. Avatec
2. Lasalocid
3. Lasalocid A
4. Ro 2 2985
5. Ro 2-2985
6. Ro 22985
7. X-537a
1. 25999-20-6
2. Bovatec
3. Avatec
4. Lasalocid A Sodium Salt
5. Lasalocid A Sodium
6. Lasalocid Sodium Salt
7. Sodium Lasalocid
8. Sodium Lasalocid A
9. Lasalocid (sodium)
10. Bovatec 150fp
11. Lasalocid A Monosodium Salt
12. Mt 2007
13. Lascaloid
14. Mls001304052
15. W2s5c71y3g
16. Lasalocid A Sodium Salt Solution
17. Smr000538906
18. Mt2007
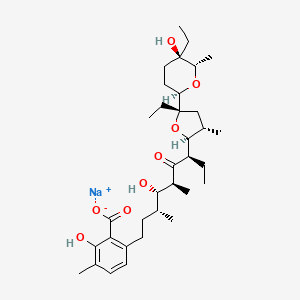
19. Sodium;6-[(3r,4s,5s,7r)-7-[(2s,3s,5s)-5-ethyl-5-[(2r,5r,6s)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-3-methyloxolan-2-yl]-4-hydroxy-3,5-dimethyl-6-oxononyl]-2-hydroxy-3-methylbenzoate
20. Bovate 20
21. Nsc243046
22. Nsc-243046
23. Unii-w2s5c71y3g
24. Lasalocid-na
25. Lasalocid-a Sodium
26. Ncgc00095029-01
27. Einecs 247-400-3
28. Cas-25999-20-6
29. Nsc 243046
30. Ro 2-2985 Sodium
31. Ionophore X-537a Sodium
32. Antibiotic X-537a Sodium
33. Dsstox_cid_25895
34. Dsstox_rid_81207
35. Dsstox_gsid_45895
36. Mls001173429
37. Mls001334026
38. Mls001334027
39. Mls006011597
40. Chembl1489769
41. Dtxsid9045895
42. Bdbm79589
43. Chebi:91848
44. Cid_6426773
45. Hy-b1071a
46. Lasalocid Sodium [mart.]
47. Hms2097f17
48. Hms2235p08
49. Np167
50. Sodium 6-[(3r,4s,5s,7r)-7-{(2s,3s,5s)-5-ethyl-5-[(2r,5r,6s)-5-ethyl-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl]-3-methyltetrahydrofuran-2-yl}-4-hydroxy-3,5-dimethyl-6-oxononyl]-2-hydroxy-3-methylbenzoate
51. Tox21_111396
52. Mfcd00078065
53. Lasalocid Sodium [green Book]
54. Akos025311506
55. Cs-5930
56. 6-(7-(5-ethyl-5-(5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)tetrahydro-3-methyl-2-furanyl)-4-hydroxy-3,5-dimethyl-6-oxononyl)-2-hydroxy-3-methylbenzoic Acid Monosodium Salt, (2r-(2alpha(2s*(3r*,4s*,5s*,7r*),3s*,5s*),5alpha,6beta))-
57. Antibiotic X-537a Sodiumlasalocid-a Sodium
58. Lasalocid A Sodium 100 Microg/ml In Methanol
59. Lasalocid A Sodium 100 Microg/ml In Acetonitrile
60. J-016217
61. Q27292230
62. Lasalocid A Sodium Salt (solution In Acetonitrile 0.2mg/2ml)
63. Lasalocid A Sodium Salt Solution, ~100 Microg/ml In Acetonitrile
64. Lasalocid-a Sodium; Ionophore X-537a Sodium; Antibiotic X-537a
65. 2,3-cresotic Acid, 6-(7-(5-ethyl-5-(5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)tetrahydro-3-methyl-2-furyl)-4-hydroxy-3,5-dimethyl-6-oxononyl)-, Monosodium Salt. (-)-
66. Benzoic Acid, 6-((3r,4s,5s,7r)-7-((2s,3s,5s)-5-ethyl-5-((2r,5r,6s)-5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)tetrahydro-3-furanyl)-4-hydroxy-3,5-dimethyl-6-oxononyl)-2-hydroxy-3-methyl-, Monosodium Salt
67. Benzoic Acid, 6-((3r,4s,5s,7r)-7-((2s,3s,5s)-5-ethyl-5-((2r,5r,6s)-5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)tetrahydro-3-methyl-2-furanyl)-4-hydroxy-3,5-dimethyl-6-oxononyl)-2-hydroxy-3-methyl-, Sodium Salt (1:1)
68. Benzoic Acid, 6-(7-(5-ethyl-5-(5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl)tetrahydro-3-methyl-2-furanyl)-4-hydroxy-3,5-dimethyl-6-oxononyl)-2-hydroxy-3-methyl-, Monosodium Salt, (2r-(2alpha(2s*(3r*,4s*,5s*,7r*),3s*,5s*),5alpha,6beta))-
69. Benzoic Acid,6-[(3r,4s,5s,7r)-7-[(2s,3s,5s)-5-ethyl-5-[(2r,5r,6s)-5-ethyltetrahydro-5-hydroxy-6-methyl-2h-pyran-2-yl]tetrahydro-3-methyl-2-furanyl]-4-hydroxy-3,5-dimethyl-6-oxononyl]-2-hydroxy-3-methy
70. Lasalocid A Sodium Salt Solution, 100 Mug/ml In Acetonitrile, Vetranal(tm), Analytical Standard
71. Sodium (1s,9s)-1,5:6,9-dianhydro-9-[(3r,5s,6s,7r)-9-(2-carboxylato-3-hydroxy-4-methylphenyl)-6-hydroxy-5,7-dimethyl-4-oxononan-3-yl]-3,4,7,8-tetradeoxy-6-ethyl-2-c-ethyl-1,8-dimethyl-d-galacto-nonitol
| Molecular Weight | 612.8 g/mol |
|---|---|
| Molecular Formula | C34H53NaO8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 13 |
| Exact Mass | 612.36381293 g/mol |
| Monoisotopic Mass | 612.36381293 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 917 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Ionophores
Chemical agents that increase the permeability of biological or artificial lipid membranes to specific ions. Most ionophores are relatively small organic molecules that act as mobile carriers within membranes or coalesce to form ion permeable channels across membranes. Many are antibiotics, and many act as uncoupling agents by short-circuiting the proton gradient across mitochondrial membranes. (See all compounds classified as Ionophores.)
Coccidiostats
Agents useful in the treatment or prevention of COCCIDIOSIS in man or animals. (See all compounds classified as Coccidiostats.)
