


1. 1847-58-1
2. Lathanol
3. Lathanol Lal
4. Nacconol Lal
5. Acetic Acid, Sulfo-, 1-dodecyl Ester, Sodium Salt
6. Sulfoacetic Acid Dodecyl Ester S-sodium Salt
7. Sodium 2-(dodecyloxy)-2-oxoethane-1-sulphonate
8. Sodium 2-(dodecyloxy)-2-oxoethanesulfonate
9. Acetic Acid, Sulfo-, Dodecyl Ester, S-sodium Salt
10. D0y70f2b9j
11. Herba Houttuyniae
12. Lathanol-lal 70
13. Dodecyl Sodium Sulfoacetate
14. Hsdb 7256
15. Dodecyl Sulfoacetate S-sodium Salt
16. Einecs 217-431-7
17. Unii-d0y70f2b9j
18. Sulfoacetic Acid 1-dodecyl Ester, Sodium Salt
19. Sulfoacetic Acid, 1-dodecyl Ester, Sodium Salt
20. Sodium Houttyfonate
21. Schembl121777
22. Dtxsid4027442
23. Bcp18547
24. Akos015900630
25. Sodium Lauryl Sulfoacetate, Aldrichcpr
26. Sodium;2-dodecoxy-2-oxoethanesulfonate
27. Ccg-267809
28. Db13157
29. Sodium Lauryl Sulfoacetate [ii]
30. Sodium Lauryl Sulfoacetate [hsdb]
31. Sodium Lauryl Sulfoacetate [inci]
32. Sodium Lauryl Sulfoacetate [vandf]
33. Sodium2-(dodecyloxy)-2-oxoethanesulfonate
34. Hy-107789
35. Sodium Lauryl Sulfoacetate [who-dd]
36. Cs-0030663
37. Ft-0657433
38. Na 2-(dodecyloxy)-2-oxoethane-1-sulphonate
39. H11025
40. W-109718
41. Q27275949
42. Acetic Acid, 2-sulfo-, Dodecyl Ester, Sodium Salt (1:1)
43. Sodium New Houttuyfonate Pound>>sodium Houttuyfonate Pound>>lathanol Lal Pound>>nacconol Lal
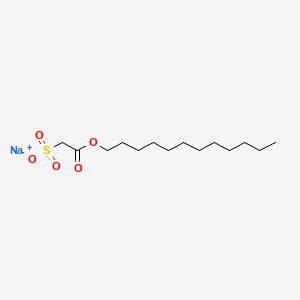
| Molecular Weight | 330.42 g/mol |
|---|---|
| Molecular Formula | C14H27NaO5S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 14 |
| Exact Mass | 330.14768941 g/mol |
| Monoisotopic Mass | 330.14768941 g/mol |
| Topological Polar Surface Area | 91.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 338 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Sodium lauryl sulfoacetate is not an active pharmacological ingredient in pharmaceutical preparations and so has no official indication.
Sodium lauryl sulfoacetate acts as a wetting agent and surfactant in pharmaceutical preparations. It is currently used in enema-type laxatives.
B05XA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Sodium lauryl Sulfoacetate can be absorbed through guinea pig skin. Two groups of 6 female weanling guinea pigs were immersed in either 0.2% aqueous sodium lauryl sulfoacetate or distilled water for 4 hr on 3 consecutive days. ... Seven blood samples were taken before and after each immersion and 24 hr after the final immersion. ....The blood concentrations of sodium lauryl sulfoacetate reached a maximum at the end of each immersion, which increased with each subsequent immersion.
Christian M, ed; J American College of Toxicology 6 (3): 261- 278 (1987)