


1. Gs-ca1
1. Gs-6207
2. 2189684-44-2
3. Gs-hiv
4. Gs-ca2
5. Gs-ca-2
6. Lenacapavir [usan]
7. Gs-ca1
8. A9a0o6fb4h
9. Gs-714207
10. Gs6207
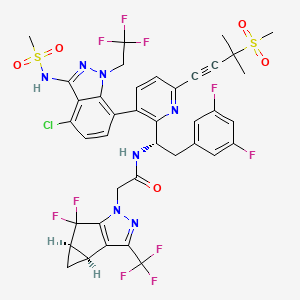
11. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonylbut-1-ynyl)pyridin-2-yl]-2-(3,5-difluorophenyl)ethyl]-2-[(2s,4r)-5,5-difluoro-9-(trifluoromethyl)-7,8-diazatricyclo[4.3.0.02,4]nona-1(6),8-dien-7-yl]acetamide
12. N-((s)-1-(3-(4-chloro-3-(methylsulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl)acetamide
13. N-[(1s)-1-(3-{4-chloro-3-[(methylsulfonyl)amino]-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl}-6-[3-methyl-3-(methylsulfonyl)but-1-yn-1-yl]pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl]-2-[(3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl]acetamide
14. Qng
15. Lenacapavir [inn]
16. Unii-a9a0o6fb4h
17. Lenacapavir [who-dd]
18. Chembl4594438
19. Schembl19875642
20. Gtpl11446
21. Ex-a5518
22. Who 11108
23. At20076
24. Hy-111964
25. Cs-0094695
26. 1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazole, N-((1s)-1-(3-(4-chloro-3-((methylsulfonyl)amino)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)-1-butyn-1-yl)-2-pyridinyl)-2-(3,5-difluorophenyl)ethyl)-5,5-difluoro-3b,4,4a,5-tetrahydro-3-(trifluoromethyl)-, (3bs,4ar)-
27. N-((1s)-1-(3-(4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)-1h-indazol-7-yl)-6-(3-(methanesulfonyl)-3-methylbut-1-yn-1-yl)pyridin-2-yl)-2-(3,5- Difluorophenyl)ethyl)-2-((3bs,4ar)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1h-cyclopropa(3,4)cyclopenta(1,2-c)pyrazol-1-yl)acetamide
28. N-[(1s)-1-[3-[4-chloro-3-(methanesulfonamido)-1-(2,2,2-trifluoroethyl)indazol-7-yl]-6-(3-methyl-3-methylsulfonyl-but-1-ynyl)-2-pyridyl]-2-(3,5-difluorophenyl)ethyl]-2-[difluoro(trifluoromethyl)[?]yl]acetamide
| Molecular Weight | 968.3 g/mol |
|---|---|
| Molecular Formula | C39H32ClF10N7O5S2 |
| XLogP3 | 6.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 13 |
| Exact Mass | 967.1435188 g/mol |
| Monoisotopic Mass | 967.1435188 g/mol |
| Topological Polar Surface Area | 175 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 2040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of human immunodeficiency virus (HIV-1) infection
* Sunlenca injection: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen (see sections 4. 2 and 5. 1).
* Sunlenca tablet: , in combination with other antiretroviral(s), is indicated for the treatment of adults with multidrug resistant HIV 1 infection for whom it is otherwise not possible to construct a suppressive anti viral regimen, for oral loading prior to administration of long-acting lenacapavir injection (see sections 4. 2 and 5. 1).
J05AX
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX31 - Lenacapavir