

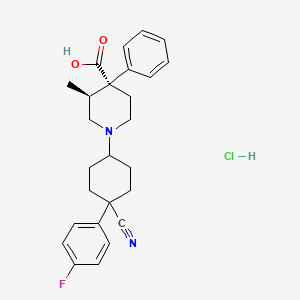

1. 1-(4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-4-piperidinecarboxylic Acid
2. Bilina
3. Lvophta
4. Levocabastine
5. Levophta
6. Livocab
7. Livostin
1. Levocabastine Hcl
2. Livostin
3. 79547-78-7
4. Livostin (tn)
5. R 50,547
6. 124xma6yei
7. Levocabastine (hydrochloride)
8. Levophta
9. (-)-trans-1-(cis-4-cyano-4-(p-fluorophenyl)cyclohexyl)-3-methyl-4-phenylisonipecotic Acid Monohydrochloride
10. Dsstox_cid_25508
11. Dsstox_rid_80921
12. Dsstox_gsid_45508
13. R-50547
14. (3s,4r)-1-((1s,4r)-4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl-4-phenylpiperidine-4-carboxylic Acid Hydrochloride
15. Levocabastine Hydrochloride [usan]
16. Unii-124xma6yei
17. (3s,4r)-cabastine Hydrochloride
18. Levocabastine Hydrochloride [usan:usp]
19. Ncgc00016939-01
20. Cas-79547-78-7
21. Schembl99969
22. Schembl99970
23. Mls002154116
24. Chembl1237102
25. Dtxsid9045508
26. Chebi:31777
27. Hms1571o19
28. Tox21_110695
29. Hy-14277a
30. Levocabastine Hydrochloride (jan/usp)
31. Tox21_110695_1
32. At25912
33. Ccg-221039
34. Levocabastine Hydrochloride [jan]
35. Ncgc00179240-04
36. 4-piperidinecarboxylic Acid, 1-(4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-, Monohydrochloride, (-)-(1(cis),3alpha,4beta)-
37. 4-piperidinecarboxylic Acid, 1-(cis-4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-, Monohydrochloride, (3s,4r)-
38. Smr001233423
39. Levocabastine Hydrochloride [mart.]
40. Levocabastine Hydrochloride [vandf]
41. Levocabastine Hydrochloride [usp-rs]
42. Levocabastine Hydrochloride [who-dd]
43. Cs-0031223
44. D01717
45. Levocabastine Hydrochloride [ep Impurity]
46. Levocabastine Hydrochloride [orange Book]
47. Levocabastine Hydrochloride [ep Monograph]
48. Levocabastine Hydrochloride [usp Impurity]
49. Levocabastine Hydrochloride [usp Monograph]
50. Levocabastine Hydrochloride, >=99% (hplc), Solid
51. Q27251370
52. Levocabastine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
53. Levocabastine For System Suitability 2, European Pharmacopoeia (ep) Reference Standard
54. Levocabastine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
55. (3s,4r)-1-[4-cyano-4-(4-fluorophenyl)cyclohexyl]-3-methyl-4-phenylpiperidine-4-carboxylic Acid;hydrochloride
56. 4-piperidinecarboxylic Acid, 1-(4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-, Monohydrochloride, (-)-(1(cis),3.alpha.,4.beta.)-
| Molecular Weight | 457.0 g/mol |
|---|---|
| Molecular Formula | C26H30ClFN2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 456.1979841 g/mol |
| Monoisotopic Mass | 456.1979841 g/mol |
| Topological Polar Surface Area | 64.3 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 681 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
