

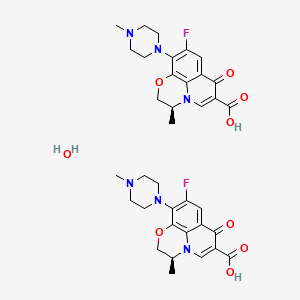

1. Anhydrous, Levofloxacin
2. Levaquin
3. Levofloxacin
4. Levofloxacin Anhydrous
5. Ofloxacin, (s)-isomer
6. Quixin
1. 138199-71-0
2. Levofloxacin Hydrate
3. 6gnt3y5lmf
4. Nofaxin
5. Quinsair
6. Volequin
7. Levaquin (tn)
8. Ofloxacin, (s)-
9. Rwj-25213
10. (-)-(s)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic Acid, Hemihydrate
11. 7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic Acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-hydrate (2:1), (s)-
12. Levofloxacin [usan]
13. Dynaquin
14. Levofloxacin (as Hemihydrate)
15. (s)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7h-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic Acid Hemihydrate
16. Unii-6gnt3y5lmf
17. Rwj 25213
18. Tavanic Hydrate
19. Cravit Hydrate
20. Iquix Hydrate
21. Quixin Hydrate
22. Levaquin Hydrate
23. Mfcd07772024
24. Levofloxacin (usp)
25. Quixin (tn)
26. Lvfx
27. Iquix (tn)
28. (s)-(-)-ofloxacine
29. (2s)-7-fluoro-2-methyl-6-(4-methylpiperazin-1-yl)-10-oxo-4-oxa-1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic Acid;hydrate
30. Levofloxacin [vandf]
31. Levofloxacin [mart.]
32. (s)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic Acid Hydrate (2:1)
33. (s)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-2h-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic Acid Hydrate(2:1)
34. Levofloxacin Hydrate (jp17)
35. Levofloxacin [usp-rs]
36. Levofloxacin Hemihidrated
37. Schembl1650602
38. Dtxsid60160533
39. Levofloxacin [orange Book]
40. Levofloxacin Hydrate [jan]
41. Levofloxacin [usp Monograph]
42. Akos015896149
43. Ks-1077
44. Levofloxacin [usan:usp:inn:ban:jan]
45. Levofloxacin Hemihydrate [who-dd]
46. 7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic Acid, 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-, Hydrate (2:1), (s)-
47. D00588
48. Levofloxacin Hemihydrate [ep Monograph]
49. Ofloxacin S-(-)-form Hemihydrate [mi]
50. 199l710
51. A886273
52. Q47495791
53. Levofloxacin, United States Pharmacopeia (usp) Reference Standard
54. 3 Inverted Exclamation Mark ,5 Inverted Exclamation Mark -difluoro-4-propylbiphenyl
55. Levofloxacin Hemihydrate, Pharmaceutical Secondary Standard; Certified Reference Material
56. (s)-(-)-ofloxacine; Levofloxacine; (-)-(s)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7h-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic Acid Hydrochloride; Levofloxacin Hemihydrate;(s)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-3,7-dihydro-2h-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic Acid Hemihydrate
57. (s)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7h-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic Acid Hemihydrate
| Molecular Weight | 740.7 g/mol |
|---|---|
| Molecular Formula | C36H42F2N6O9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 4 |
| Exact Mass | 740.29813326 g/mol |
| Monoisotopic Mass | 740.29813326 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 634 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Quinsair is indicated for the management of chronic pulmonary infections due to Pseudomonas aeruginosa in adult patients with cystic fibrosis.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of cystic fibrosis
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Cytochrome P-450 CYP1A2 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inhibitors.)
J01MA12