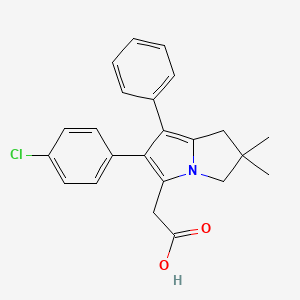

1. (2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1h-pyrrolizine-5-yl)acetic Acid
2. Ml 3000
3. Ml-3000
1. 156897-06-2
2. Ml-3000
3. Licofelone [inn]
4. Ml 3000
5. 2-[2-(4-chlorophenyl)-6,6-dimethyl-1-phenyl-5,7-dihydropyrrolizin-3-yl]acetic Acid
6. P5t6bys22y
7. (6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizin-5-yl)acetic Acid
8. [6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizin-5-yl]acetic Acid
9. Chembl300982
10. 2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizin-5-yl)acetic Acid
11. Lcf
12. 2-[6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizin-5-yl]acetic Acid
13. Unii-p5t6bys22y
14. Brn 6823674
15. 6-(4-chlorophenyl)-2,3-dihydro-2,2-dimethyl-7-phenyl-1h-pyrrolizine-5-acetic Acid
16. 2,2-dimethyl-6-(4-chlorophenyl-7-phenyl-2,3-dihydro-1h-pyrrolizine-5-yl)acetic Acid
17. 2,3-dihydro-6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-1h-pyrrolizine-5-acetic Acid
18. Licofelone [mi]
19. Licofelone [mart.]
20. Mls006011224
21. Schembl237833
22. Dtxsid40166154
23. Hms3655d21
24. Bcp06598
25. Hy-b1452
26. Zinc3805769
27. Bdbm50038649
28. Ml3000
29. S2121
30. Akos027250758
31. Db04725
32. Sb19520
33. (6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizine-5-yl)-acetic Acid
34. 1h-pyrrolizine-5-acetic Acid, 2,3-dihydro-6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-
35. Ncgc00250374-01
36. Ncgc00250374-02
37. Ac-33111
38. As-73938
39. Smr004702985
40. Cs-0013159
41. Ft-0670787
42. Sw219543-1
43. Ab01566204_02
44. 897l062
45. A809783
46. J-009352
47. Q3832015
48. [2-(4-chloro-phenyl)-6,6-dimethyl-1-phenyl-6,7-dihydro-5h-pyrrolizin-3-yl]-acetic Acid
49. 1h-pyrrolizine-5-acetic Acid, 6-(4-chlorophenyl)-2,3-dihydro-2,2-dimethyl-7-phenyl-
50. 6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-dihydro-1h-pyrrolizin-5-ylacetic Acid
| Molecular Weight | 379.9 g/mol |
|---|---|
| Molecular Formula | C23H22ClNO2 |
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 379.1339066 g/mol |
| Monoisotopic Mass | 379.1339066 g/mol |
| Topological Polar Surface Area | 42.2 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 537 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the management of osteoarthritis.
Licofelone belongs to a novel class of dual-acting anti-inflammatory drugs called COX/LO inhibitors. This group of drugs simultaneously inhibits the enzymes cyclooxygenase (COX) and 5-lipoxygenase (LO).
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Lipoxygenase Inhibitors
Compounds that bind to and inhibit that enzymatic activity of LIPOXYGENASES. Included under this category are inhibitors that are specific for lipoxygenase subtypes and act to reduce the production of LEUKOTRIENES. (See all compounds classified as Lipoxygenase Inhibitors.)
Licofelone has known human metabolites that include (2S,3S,4S,5R)-6-[2-[2-(4-chlorophenyl)-6,6-dimethyl-1-phenyl-5,7-dihydropyrrolizin-3-yl]acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid, Licofelone M2, and Licofelone M4.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Licofelone, through combined 5-LOX/COX-inhibition, reduces levels of inflammatory prostaglandins and leukotrienes.
