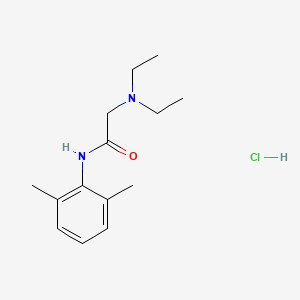

1. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide
2. 2-2etn-2mephacn
3. Dalcaine
4. Lidocaine
5. Lidocaine Carbonate
6. Lidocaine Carbonate (2:1)
7. Lidocaine Hydrocarbonate
8. Lidocaine Monoacetate
9. Lidocaine Monohydrochloride
10. Lidocaine Monohydrochloride, Monohydrate
11. Lidocaine Sulfate (1:1)
12. Octocaine
13. Xylesthesin
14. Xylocaine
15. Xylocitin
16. Xyloneural
1. 73-78-9
2. Lidocaine Hcl
3. Lidothesin
4. Xyloneural
5. Lignocaine Hydrochloride
6. Lidocaine (hydrochloride)
7. Lidocaton
8. Xylocard
9. Lidocaine Hydrochloride Anhydrous
10. Lidopen
11. Acetamide, 2-(diethylamino)-n-(2,6-dimethylphenyl)-, Monohydrochloride
12. Lta Ii Kit
13. Laryng-o-jet Kit
14. Ec2cnf7xfp
15. Linocaine Hydrochloride
16. Mls000069665
17. Laryngotracheal Anesthesia Kit
18. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide Hydrochloride
19. 2-diethylamino-2',6'-acetoxylidide Hydrochloride
20. Alphacaine Hydrochloride
21. Nsc-757420
22. Smr000058468
23. Xylocaine (tn)
24. Alpha-diethylamino-2,6-acetoxylidine Hydrochloride
25. Laryng-o-jet
26. Omega-diethylamino-2,6-dimethylacetanilide Hydrochloride
27. Xilina Hydrochloride
28. 73-78-9 (hcl); 6108-05-0 (monohydrate)
29. Rucaina Hydrochloride
30. Xycaine Hydrochloride
31. Xylotox Hydrochloride
32. Duncaine Hydrochloride
33. Isicaine Hydrochloride
34. Lidocain Hydrochloride
35. Anestacon Hydrochloride
36. Gravocain Hydrochloride
37. Leostesin Hydrochloride
38. Xylocitin Hydrochloride
39. Lidothesin Hydrochloride
40. Xylestesin Hydrochloride
41. Glydo
42. Lidocaine Viscous
43. Pediatric Lta Kit
44. Chebi:50512
45. Xylocaine Preservative Free
46. Sr-01000000189
47. Unii-ec2cnf7xfp
48. Einecs 200-803-8
49. Xylocaine 4% Preservative Free
50. Xylocaine 5% W/ Glucose 7.5%
51. Lidocaine Hydrochloride Viscous
52. S 202
53. V 262
54. Xylocaine 1.5% W/ Dextrose 7.5%
55. Dalcaine (tn)
56. Prestwick_296
57. N-(diethylaminoacetyl)-2,6-dimethylaniline Hydrochloride
58. Lidoca Ne Hydrochloride
59. Lidocaine Hydrochloride Preservative Free
60. Opera_id_351
61. Lidocaine Hydrochloride In Plastic Container
62. Lidocaine Hydrochloride 0.2% In Dextrose 5%
63. Lidocaine Hydrochloride 0.4% In Dextrose 5%
64. Lidocaine Hydrochloride 5% And Dextrose 7.5%
65. Spectrum1500689
66. Chembl541521
67. Dtxsid4058782
68. Hy-b0185a
69. 2',6'-acetoxylidide, 2-(diethylamino)-, Monohydrochloride
70. Hms1568i21
71. Hms1921c22
72. Lidocaine Hydrochloride Preservative Free In Plastic Container
73. Pharmakon1600-01500689
74. Lidocaine Hydrochloride (jan/usp)
75. 2',6'-acetoxylidide, 2-(diethylamino)-, Hydrochloride
76. Bcp30473
77. Lidocaine Hydrochloride 0.2% In Dextrose 5% In Plastic Container
78. Lidocaine Hydrochloride 0.4% In Dextrose 5% In Plastic Container
79. Lidocaine Hydrochloride 0.8% In Dextrose 5% In Plastic Container
80. Tox21_500669
81. Ccg-39281
82. Lidocaine Hydrochloride [jan]
83. Lidocaine Hydrochloride 0.1% And Dextrose 5% In Plastic Container
84. Lidocaine Hydrochloride 0.2% And Dextrose 5% In Plastic Container
85. Lidocaine Hydrochloride 0.4% And Dextrose 5% In Plastic Container
86. Lidocaine Hydrochloride 0.8% And Dextrose 5% In Plastic Container
87. Nsc757420
88. S4667
89. (unlabeled)lidocaine-d6 Hydrochloride
90. Akos015889456
91. Cs-3888
92. Lp00669
93. Nsc 757420
94. Sb19119
95. Anhydrous Lidocaine Hydrochloride
96. Lidocaine Hydrochloride [who-dd]
97. Ncgc00094030-01
98. Ncgc00094030-02
99. Ncgc00094030-03
100. Ncgc00094030-04
101. Ncgc00094030-05
102. Ncgc00261354-01
103. Ac-11712
104. As-35171
105. Eu-0100669
106. Lignocaine Hydrochloride Pound>>lidocaine Hcl
107. A16132
108. D02086
109. L 5647
110. Lidocaine Hydrochloride Anhydrous [mart.]
111. A837924
112. Q-201304
113. Sr-01000000189-3
114. Sr-01000000189-9
115. Q27122094
116. 2-(diethylamino)-2',6'-acetoxylidide Monohydrochloride
117. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide;hydrochloride
118. 2-(diethylamino)-n-(2,6-dimethylphenyl)ethanamide Hydrochloride
119. Acetamide, 2-(diethylamino)-n-(2,6-dimethylphenyl)-, Hydrochloride (1:1)
| Molecular Weight | 270.80 g/mol |
|---|---|
| Molecular Formula | C14H23ClN2O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 270.1498911 g/mol |
| Monoisotopic Mass | 270.1498911 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 228 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 18 | |
|---|---|
| Drug Name | Laryng-o-jet kit |
| Drug Label | DESCRIPTIONLidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains lidocaine hydrochloride, which is che... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 4% |
| Market Status | Prescription |
| Company | Intl Medication |
| 2 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Jelly; Injectable; Solution |
| Route | Injection; Oral; Topical |
| Strength | 0.5%; 1%; 20%; 2%; 4% |
| Market Status | Prescription |
| Company | Wockhardt; Igi Labs; Hospira; Roxane; Watson Labs; Intl Medication; Hi Tech Pharma; Vintage; Luitpold; Agila Speclts; Akorn |
| 3 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride in plastic container |
| Drug Label | Each mL contains:Lidocaine Hydrochloride . . . . . . . . . . 20 mg (2%)Inactive ingredients: flavoring, methylparaben, propylparaben, sodium carboxymethylcellulose, and sodium saccharin in an aqueous solution.Lidocaine Viscous (Lidocaine Hydrochlorid... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5%; 1%; 2% |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Usa |
| 4 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride preservative free |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 20%; 1.5%; 2%; 4% |
| Market Status | Prescription |
| Company | Hospira; Aurobindo Pharma; Intl Medication; Fresenius Kabi Usa; Agila Speclts |
| 5 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride preservative free in plastic container |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 2% |
| Market Status | Prescription |
| Company | Hospira |
| 6 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride viscous |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Vintage |
| 7 of 18 | |
|---|---|
| Drug Name | Lidocaine viscous |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Roxane |
| 8 of 18 | |
|---|---|
| Drug Name | Lta ii kit |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 4% |
| Market Status | Prescription |
| Company | Hospira |
| 9 of 18 | |
|---|---|
| Drug Name | Xylocaine preservative free |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 20%; 2%; 4% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 10 of 18 | |
|---|---|
| Drug Name | Laryng-o-jet kit |
| Drug Label | DESCRIPTIONLidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP (Viscous) 2% contains lidocaine hydrochloride, which is che... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 4% |
| Market Status | Prescription |
| Company | Intl Medication |
| 11 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Jelly; Injectable; Solution |
| Route | Injection; Oral; Topical |
| Strength | 0.5%; 1%; 20%; 2%; 4% |
| Market Status | Prescription |
| Company | Wockhardt; Igi Labs; Hospira; Roxane; Watson Labs; Intl Medication; Hi Tech Pharma; Vintage; Luitpold; Agila Speclts; Akorn |
| 12 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride in plastic container |
| Drug Label | Each mL contains:Lidocaine Hydrochloride . . . . . . . . . . 20 mg (2%)Inactive ingredients: flavoring, methylparaben, propylparaben, sodium carboxymethylcellulose, and sodium saccharin in an aqueous solution.Lidocaine Viscous (Lidocaine Hydrochlorid... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5%; 1%; 2% |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Usa |
| 13 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride preservative free |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 20%; 1.5%; 2%; 4% |
| Market Status | Prescription |
| Company | Hospira; Aurobindo Pharma; Intl Medication; Fresenius Kabi Usa; Agila Speclts |
| 14 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride preservative free in plastic container |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 2% |
| Market Status | Prescription |
| Company | Hospira |
| 15 of 18 | |
|---|---|
| Drug Name | Lidocaine hydrochloride viscous |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Vintage |
| 16 of 18 | |
|---|---|
| Drug Name | Lidocaine viscous |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Roxane |
| 17 of 18 | |
|---|---|
| Drug Name | Lta ii kit |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 4% |
| Market Status | Prescription |
| Company | Hospira |
| 18 of 18 | |
|---|---|
| Drug Name | Xylocaine preservative free |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1%; 20%; 2%; 4% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)