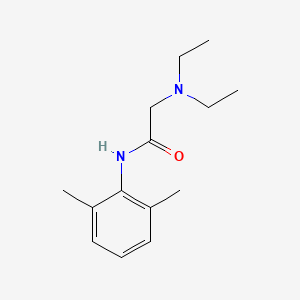

1. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide
2. 2-2etn-2mephacn
3. Dalcaine
4. Lidocaine Carbonate
5. Lidocaine Carbonate (2:1)
6. Lidocaine Hydrocarbonate
7. Lidocaine Hydrochloride
8. Lidocaine Monoacetate
9. Lidocaine Monohydrochloride
10. Lidocaine Monohydrochloride, Monohydrate
11. Lidocaine Sulfate (1:1)
12. Lignocaine
13. Octocaine
14. Xylesthesin
15. Xylocaine
16. Xylocitin
17. Xyloneural
1. 137-58-6
2. Lignocaine
3. Xylocaine
4. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide
5. Lidoderm
6. Alphacaine
7. Anestacon
8. Esracaine
9. Xylestesin
10. Duncaine
11. Cappicaine
12. Gravocain
13. Isicaina
14. L-caine
15. Leostesin
16. Maricaine
17. Xylocain
18. Xylocitin
19. Solcain
20. Isicaine
21. Rucaina
22. Xilina
23. Xycaine
24. Xylotox
25. Cito Optadren
26. Lida-mantle
27. 2-(diethylamino)-2',6'-acetoxylidide
28. Dentipatch
29. Dalcaine
30. Xyloneural (free Base)
31. Cuivasil
32. Jetocaine
33. Lidocainum
34. Remicaine
35. Xilocaina
36. 2-diethylamino-n-(2,6-dimethylphenyl)acetamide
37. Acetamide, 2-(diethylamino)-n-(2,6-dimethylphenyl)-
38. Diethylaminoaceto-2,6-xylidide
39. Ela-max
40. Ztlido
41. 2',6'-acetoxylidide, 2-(diethylamino)-
42. Alpha-diethylamino-2,6-dimethylacetanilide
43. Lidocaton
44. Alfa-dietilamino-2,6-dimetilacetanilide
45. Chebi:6456
46. Xylocaine (tn)
47. Chembl79
48. Nsc-40030
49. Lidopen
50. N-(2,6-dimethylphenyl)-n~2~,n~2~-diethylglycinamide
51. Mls000069724
52. Algrx 3268
53. Algrx-3268
54. Lignocainum
55. Xllina
56. N-(2,6-dimethylphenyl)-n(2),n(2)-diethylglycinamide
57. Lidocaine (van)
58. .alpha.-diethylaminoaceto-2,6-xylidide
59. Ncgc00015611-10
60. Xilocaina [italian]
61. Dilocaine
62. Lidocaina
63. Smr000058189
64. .alpha.-(diethylamino)-2,6-acetoxylidide
65. Lidocaine Base
66. .alpha.-diethylamino-2,6-dimethylacetanilide
67. .omega.-diethylamino-2,6-dimethylacetanilide
68. 98pi200987
69. Lidocainum [inn-latin]
70. Dsstox_cid_25166
71. Dsstox_rid_80716
72. Dsstox_gsid_45166
73. Lidocaina [inn-spanish]
74. N-(2,6-dimethylphenyl)-n2,n2-diethylglycinamide
75. 2-(diethylamino)-n-(2,6-dimethylphenyl)ethanamide
76. Embolex
77. Versatis
78. Ztilido
79. 2-(diethylamino)-n-(2,6-dimethylphenyl)-acetamide
80. Dentipatch (tn)
81. 91484-71-8
82. Cas-137-58-6
83. Lqz
84. Hsdb 3350
85. Einecs 205-302-8
86. Mfcd00026733
87. Nsc 40030
88. Alfa-dietilamino-2,6-dimetilacetanilide [italian]
89. Brn 2215784
90. Qualigens
91. Xyline
92. Lignocaine Base
93. Lidocainehclh2o
94. Lidocaine [usp:inn:ban:jan]
95. Lidocaine, Powder
96. Unii-98pi200987
97. N1-(2,6-dimethylphenyl)-n2,n2-diethylglycinamide
98. Zingo (salt/mix)
99. Cds1_000283
100. Lidocaine (alphacaine)
101. Spectrum_001118
102. Lidothesin (salt/mix)
103. Xyloneural (salt/mix)
104. Lidocaine [inn]
105. Lidocaine [jan]
106. Opera_id_385
107. Lidocaine [mi]
108. Lidocaine [hsdb]
109. Lidocaine [inci]
110. Maybridge1_002571
111. Prestwick0_000050
112. Prestwick1_000050
113. Prestwick2_000050
114. Prestwick3_000050
115. Spectrum2_001343
116. Spectrum3_001392
117. Spectrum4_000070
118. Spectrum5_001549
119. Lidocaine [vandf]
120. Lopac-l-5647
121. Lidaform Hc (salt/mix)
122. Epitope Id:116205
123. Lidocaine [mart.]
124. Lidamantle Hc (salt/mix)
125. 2', 2-(diethylamino)-
126. Lidocaine [usp-rs]
127. Lidocaine [who-dd]
128. Lidocaine [who-ip]
129. Neosporin Plus (salt/mix)
130. Lopac0_000669
131. Schembl15689
132. Bspbio_000179
133. Bspbio_001359
134. Bspbio_003004
135. Kbiogr_000079
136. Kbiogr_000599
137. Kbioss_000079
138. Kbioss_001598
139. 2-diethylamino-n-(2,6-dimethyl-phenyl)-acetamide
140. 4-12-00-02538 (beilstein Handbook Reference)
141. Mls000758263
142. Mls001074177
143. Mls001423964
144. Bidd:gt0342
145. Diethylaminoacet-2,6-xylidide
146. Divk1c_000174
147. Divk1c_001323
148. Lidocaine, Analytical Standard
149. Spbio_001525
150. Spbio_002100
151. Emla Component Lidocaine
152. Lidocaine (jp17/usp/inn)
153. Lidocaine [green Book]
154. Bpbio1_000197
155. Gtpl2623
156. Lidocaine [orange Book]
157. Dtxsid1045166
158. Lidocaine [ep Monograph]
159. Schembl17967359
160. Hms548m19
161. Kbio1_000174
162. Kbio2_000079
163. Kbio2_001598
164. Kbio2_002647
165. Kbio2_004166
166. Kbio2_005215
167. Kbio2_006734
168. Kbio3_000157
169. Kbio3_000158
170. Kbio3_002224
171. Oraqix Component Lidocaine
172. Synera Component Lidocaine
173. Zinc20237
174. Lidocaine [usp Monograph]
175. Lidocaine 1.0 Mg/ml In Methanol
176. Lidocainum [who-ip Latin]
177. Ninds_000174
178. Bio1_000379
179. Bio1_000868
180. Bio1_001357
181. Bio2_000079
182. Bio2_000559
183. Hms1791d21
184. Hms1989d21
185. Hms2051c21
186. Hms2089e15
187. Hms2235o14
188. Hms3371j04
189. Hms3393c21
190. Hms3428o07
191. Hms3651g09
192. Lidocaine Component Of Emla
193. Fortacin Component Lidocaine
194. Amy25560
195. Bcp09081
196. Hy-b0185
197. Nsc40030
198. Lidocaine Component Of Oraqix
199. Lidocaine Component Of Synera
200. Tox21_110183
201. 2-diethylamino-2',6'-acetoxylidide
202. Bdbm50017662
203. Lanabiotic Component Lidocaine
204. Nsc789222
205. S1357
206. Stk552033
207. Akos001026768
208. Lidocain Component Of Fortacine
209. Tox21_110183_1
210. Ccg-100824
211. Cs-2070
212. Db00281
213. Nc00074
214. Nsc-789222
215. Rocephin Kit Component Lidocaine
216. Sb19118
217. Sdccgsbi-0050648.p005
218. Wln: 2n2 & 1vmr B1 F1
219. .alpha.-diethylamino-2,6-acetoxylidide
220. Cas-73-78-9
221. Idi1_000174
222. Idi1_033829
223. Lidocaine Component Of Lanabiotic
224. Ncgc00015611-01
225. Ncgc00015611-02
226. Ncgc00015611-03
227. Ncgc00015611-04
228. Ncgc00015611-05
229. Ncgc00015611-06
230. Ncgc00015611-07
231. Ncgc00015611-08
232. Ncgc00015611-09
233. Ncgc00015611-11
234. Ncgc00015611-12
235. Ncgc00015611-13
236. Ncgc00015611-14
237. Ncgc00015611-15
238. Ncgc00015611-16
239. Ncgc00015611-18
240. Ncgc00015611-31
241. Ncgc00022176-05
242. Ncgc00022176-06
243. Ncgc00022176-07
244. Ncgc00022176-08
245. Ncgc00022176-09
246. Ac-10282
247. As-13718
248. Sy052029
249. 2-(diethylamino)-2'',6''-acetoxylidide
250. Lidocaine Component Of Rocephin Kit
251. Sbi-0050648.p004
252. Diethylamino-2,6-dimethylacetanilide
253. Lignocaine Base 100 Microg/ml In Methanol
254. Ab00053581
255. L0156
256. Sw196598-4
257. A18187
258. C07073
259. D00358
260. M06299
261. Ab00053581-27
262. Ab00053581-28
263. Ab00053581_29
264. Ab00053581_30
265. A833036
266. Q216935
267. (2,6-dimethylphenyl)carbamoylmethyl-diethyl-azanium
268. N1-(2,6-dimethylphenyl)-2-(diethylamino)acetamide
269. W-108233
270. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide #
271. Brd-k52662033-001-02-6
272. Brd-k52662033-003-05-5
273. Brd-k52662033-003-14-7
274. Z55135799
275. Lidocaine, British Pharmacopoeia (bp) Reference Standard
276. Lidocaine, European Pharmacopoeia (ep) Reference Standard
277. N~1~-(2,6-dimethylphenyl)-n~2~,n~2~-diethylglycinamide
278. Lidocaine, United States Pharmacopeia (usp) Reference Standard
279. 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide Hydrate Hydrochloride
280. Lidocaine, Pharmaceutical Secondary Standard; Certified Reference Material
281. Lidocaine Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
1. Lignocaine
| Molecular Weight | 234.34 g/mol |
|---|---|
| Molecular Formula | C14H22N2O |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 234.173213330 g/mol |
| Monoisotopic Mass | 234.173213330 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 228 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Lidocaine |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local, Antiarrhythmic, Group IB |
| Drug Label | Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains a local anesthetic agent and is administered topically. Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains lidocaine HCl, which is chemically designated as... |
| Active Ingredient | Lidocaine |
| Dosage Form | Ointment; Patch |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Taro; Fougera; Watson Labs; Novocol |
| Patent | 5741510; 5827529 |
| 2 of 12 | |
|---|---|
| Drug Name | Lidoderm |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local |
| Drug Label | DESCRIPTIONLIDODERM (lidocaine patch 5%) is comprised of an adhesive material containing 5% lidocaine, which is applied to a non-woven polyester felt backing and covered with a polyethylene terephthalate (PET) film release liner. The release liner is... |
| Active Ingredient | Lidocaine |
| Dosage Form | Patch |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Teikoku Pharma Usa |
| Patent | 8540665; 5630796; 5899880; 6004286; 6881200 |
| 3 of 12 | |
|---|---|
| Drug Name | Octocaine |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local |
| Active Ingredient | lidocaine hydrochloride; Epinephrine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/ml; 2%; 0.01mg/ml |
| Market Status | Prescription |
| Company | Septodont |
| 4 of 12 | |
|---|---|
| Drug Name | Xylocaine |
| PubMed Health | Lidocaine |
| Drug Label | XYLOCAINE - lidocaine hydrochloride injection, solutionXYLOCAINE - lidocaine hydrochloride and epinephrine bitartrate injection, solutionXYLOCAINE MPF - lidocaine hydrochloride injection, solutionXYLOCAINE - lidocaine hydrochloride and epinephrine bi... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Jelly; Injectable |
| Route | Injection; Topical |
| Strength | 0.5%; 1%; 1.5%; 2% |
| Market Status | Prescription |
| Company | Oak Pharms; Fresenius Kabi Usa |
| 5 of 12 | |
|---|---|
| Drug Name | Xylocaine viscous |
| PubMed Health | Lidocaine |
| Drug Label | Zingo (lidocaine hydrochloride monohydrate)powder intradermal injectionsystemcontains 0.5 mg of sterile lidocaine hydrochloride monohydrate.The chemical name is 2-diethylamino-2',6'-acetoxylidide, monohydrochloride, monohydrate. Themolecul... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 6 of 12 | |
|---|---|
| Drug Name | Zingo |
| PubMed Health | Lidocaine |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | System |
| Route | Intradermal |
| Strength | 0.5mg |
| Market Status | Prescription |
| Company | Powder Pharms |
| 7 of 12 | |
|---|---|
| Drug Name | Lidocaine |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local, Antiarrhythmic, Group IB |
| Drug Label | Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains a local anesthetic agent and is administered topically. Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains lidocaine HCl, which is chemically designated as... |
| Active Ingredient | Lidocaine |
| Dosage Form | Ointment; Patch |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Taro; Fougera; Watson Labs; Novocol |
| Patent | 5741510; 5827529 |
| 8 of 12 | |
|---|---|
| Drug Name | Lidoderm |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local |
| Drug Label | DESCRIPTIONLIDODERM (lidocaine patch 5%) is comprised of an adhesive material containing 5% lidocaine, which is applied to a non-woven polyester felt backing and covered with a polyethylene terephthalate (PET) film release liner. The release liner is... |
| Active Ingredient | Lidocaine |
| Dosage Form | Patch |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Teikoku Pharma Usa |
| Patent | 8540665; 5630796; 5899880; 6004286; 6881200 |
| 9 of 12 | |
|---|---|
| Drug Name | Octocaine |
| PubMed Health | Lidocaine |
| Drug Classes | Anesthetic, Local |
| Active Ingredient | lidocaine hydrochloride; Epinephrine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/ml; 2%; 0.01mg/ml |
| Market Status | Prescription |
| Company | Septodont |
| 10 of 12 | |
|---|---|
| Drug Name | Xylocaine |
| PubMed Health | Lidocaine |
| Drug Label | XYLOCAINE - lidocaine hydrochloride injection, solutionXYLOCAINE - lidocaine hydrochloride and epinephrine bitartrate injection, solutionXYLOCAINE MPF - lidocaine hydrochloride injection, solutionXYLOCAINE - lidocaine hydrochloride and epinephrine bi... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Jelly; Injectable |
| Route | Injection; Topical |
| Strength | 0.5%; 1%; 1.5%; 2% |
| Market Status | Prescription |
| Company | Oak Pharms; Fresenius Kabi Usa |
| 11 of 12 | |
|---|---|
| Drug Name | Xylocaine viscous |
| PubMed Health | Lidocaine |
| Drug Label | Zingo (lidocaine hydrochloride monohydrate)powder intradermal injectionsystemcontains 0.5 mg of sterile lidocaine hydrochloride monohydrate.The chemical name is 2-diethylamino-2',6'-acetoxylidide, monohydrochloride, monohydrate. Themolecul... |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 2% |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 12 of 12 | |
|---|---|
| Drug Name | Zingo |
| PubMed Health | Lidocaine |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | System |
| Route | Intradermal |
| Strength | 0.5mg |
| Market Status | Prescription |
| Company | Powder Pharms |
Anesthetics, Local; Anti-Arrhythmia Agents; Voltage-Gated Sodium Channel Blockers
National Library of Medicine's Medical Subject Headings. Lidocaine. Online file (MeSH, 2014). Available from, as of August 28, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Lidocaine hydrochloride is used for infiltration anesthesia and for nerve block techniques including peripheral, sympathetic, epidural (including caudal), and spinal block anesthesia. /Included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3340
Lidocaine has been administered intraperitoneally for anesthesia of the peritoneum and pelvic viscera. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3340
Lidocaine is considered an alternative antiarrhythmic agent to amiodarone in the treatment of cardiac arrest secondary to ventricular fibrillation or pulseless ventricular tachycardia resistant to cardiopulmonary resuscitation (CPR), electrical cardioversion (e.g., after 2 to 3 shocks) and a vasopressor (epinephrine, vasopressin). /Included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1694
For more Therapeutic Uses (Complete) data for LIDOCAINE (21 total), please visit the HSDB record page.
WARNING: Life-threatening and fatal events in infants and young children. Postmarketing cases of seizures, cardiopulmonary arrest, and death in patients under the age of 3 years have been reported with use of Xylocaine 2% Viscous Solution when it was not administered in strict adherence to the dosing and administration recommendations. In the setting of teething pain, Xylocaine 2% Viscous Solution should generally not be used. For other conditions, the use of the product in patients less than 3 years of age should be limited to those situations where safer alternatives are not available or have been tried but failed. To decrease the risk of serious adverse events with use of Xylocaine 2% Viscous Solution, instruct caregivers to strictly adhere to the prescribed dose and frequency of administration and store the prescription bottle safely out of reach of children.
FDA; Prescribing Information for 2% Xylocaine Viscous (Lidocaine Hydrochloride) Solution (September 2014). Available from, as of November 2014: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/009470s025lbl.pdf
Life-threatening adverse effects (e.g., irregular heart beat, seizures, breathing difficulties, coma, death) may occur when topical anesthetics are applied to a large area of skin, when the area of application is covered with an occlusive dressing, if a large amount of topical anesthetic is applied, if the anesthetic is applied to irritated or broken skin, or if the skin temperature increases (from exercise or use of a heating pad).101 102 When applied in such a manner, the amount of anesthetic that is absorbed systemically is unpredictable and the plasma concentrations achieved may be high enough to cause life-threatening adverse effects.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3341-2
The Food and Drug Administration (FDA) has reviewed 35 reports of chondrolysis (necrosis and destruction of cartilage) in patients given continuous intra-articular infusions of local anesthetics with elastomeric infusion devices to control post-surgical pain. The significance of this injury to otherwise healthy young adults warrants notification to health care professionals. The local anesthetics (with and without epinephrine) were infused for extended periods of time (48 to 72 hours) directly into the intra-articular space using an elastomeric pump. Chondrolysis was diagnosed within a median of 8.5 months after the infusion. Almost all of the reported cases of chondrolysis (97%) occurred following shoulder surgeries. Joint pain, stiffness, and loss of motion were reported as early as the second month after receiving the infusion. In more than half of these reports, the patients required additional surgery, including arthroscopy or arthroplasty (joint replacement). It is not known which specific factor or combination of factors contributed to the development of chondrolysis in these cases. The infused local anesthetic drugs, the device materials, and/or other sources may have resulted in the development of chondrolysis. It is important to note that single intra-articular injections of local anesthetics in orthopedic procedures have been used for many years without any reported occurrence of chondrolysis. Local anesthetics are approved as injections for the production of local or regional anesthesia or analgesia. Neither local anesthetics nor infusion devices are approved for an indication of continuous intra-articular infusion.
FDA; Information for Healthcare Professionals: Chondrolysis Reported with Continuously Infused Local Anesthetics (Marketed as Bupivacaine, Chlorprocaine, Lidocaine, Mepivacaine, Procaine and Ropivacaine) (February 16, 2010). Available from, as of November 24, 2014: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190302.htm
Local anesthetics should only be administered by clinicians who are experienced in the diagnosis and management of dose-related toxicities and other acute emergencies associated with these agents. Resuscitative equipment, oxygen, drugs, and personnel required for treatment of adverse reactions should be immediately available when lidocaine is administered. Proper positioning of the patient is extremely important in spinal anesthesia.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3341
For more Drug Warnings (Complete) data for LIDOCAINE (31 total), please visit the HSDB record page.
Two fatal cases of deliberate self poisoning with lignocaine are reported, one by oral ingestion and one by iv injection. Post-mortem blood lignocaine concn were 40 and 53 mg/L, respectively.
PMID:2680899 Dawling S et al; Hum Toxicol 8 (5): 389-92 (1989)
Topical application of 25 g of lidocaine base twice daily led to death from cardiorespiratory arrest.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1199
Lidocaine is an anesthetic of the amide group indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by peripheral nerve block techniques such as brachial plexus and intercostal and by central neural techniques such as lumbar and caudal epidural blocks.
FDA Label
Excessive blood levels of lidocaine can cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. With central neural blockade these changes may be attributable to the block of autonomic fibers, a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system, and/or the beta-adrenergic receptor stimulating action of epinephrine when present. The net effect is normally a modest hypotension when the recommended dosages are not exceeded. In particular, such cardiac effects are likely associated with the principal effect that lidocaine elicits when it binds and blocks sodium channels, inhibiting the ionic fluxes required for the initiation and conduction of electrical action potential impulses necessary to facilitate muscle contraction. Subsequently, in cardiac myocytes, lidocaine can potentially block or otherwise slow the rise of cardiac action potentials and their associated cardiac myocyte contractions, resulting in possible effects like hypotension, bradycardia, myocardial depression, cardiac arrhythmias, and perhaps cardiac arrest or circulatory collapse. Moreover, lidocaine possesses a dissociation constant (pKa) of 7.7 and is considered a weak base. As a result, about 25% of lidocaine molecules will be un-ionized and available at the physiological pH of 7.4 to translocate inside nerve cells, which means lidocaine elicits an onset of action more rapidly than other local anesthetics that have higher pKa values. This rapid onset of action is demonstrated in about one minute following intravenous injection and fifteen minutes following intramuscular injection. The administered lidocaine subsequently spreads rapidly through the surrounding tissues and the anesthetic effect lasts approximately ten to twenty minutes when given intravenously and about sixty to ninety minutes after intramuscular injection. Nevertheless, it appears that the efficacy of lidocaine may be minimized in the presence of inflammation. This effect could be due to acidosis decreasing the amount of un-ionized lidocaine molecules, a more rapid reduction in lidocaine concentration as a result of increased blood flow, or potentially also because of increased production of inflammatory mediators like peroxynitrite that elicit direct actions on sodium channels.
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
N01BB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N01BB02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N01BB02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N01BB02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C - Cardiovascular system
C01 - Cardiac therapy
C01B - Antiarrhythmics, class i and iii
C01BB - Antiarrhythmics, class ib
C01BB01 - Lidocaine
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AD - Local anesthetics
C05AD01 - Lidocaine
D - Dermatologicals
D04 - Antipruritics, incl. antihistamines, anesthetics, etc.
D04A - Antipruritics, incl. antihistamines, anesthetics, etc.
D04AB - Anesthetics for topical use
D04AB01 - Lidocaine
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BB - Amides
N01BB02 - Lidocaine
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AD - Anesthetics, local
R02AD02 - Lidocaine
S - Sensory organs
S01 - Ophthalmologicals
S01H - Local anesthetics
S01HA - Local anesthetics
S01HA07 - Lidocaine
S - Sensory organs
S02 - Otologicals
S02D - Other otologicals
S02DA - Analgesics and anesthetics
S02DA01 - Lidocaine
Absorption
In general, lidocaine is readily absorbed across mucous membranes and damaged skin but poorly through intact skin. The agent is quickly absorbed from the upper airway, tracheobronchial tree, and alveoli into the bloodstream. And although lidocaine is also well absorbed across the gastrointestinal tract the oral bioavailability is only about 35% as a result of a high degree of first-pass metabolism. After injection into tissues, lidocaine is also rapidly absorbed and the absorption rate is affected by both vascularity and the presence of tissue and fat capable of binding lidocaine in the particular tissues. The concentration of lidocaine in the blood is subsequently affected by a variety of aspects, including its rate of absorption from the site of injection, the rate of tissue distribution, and the rate of metabolism and excretion. Subsequently, the systemic absorption of lidocaine is determined by the site of injection, the dosage given, and its pharmacological profile. The maximum blood concentration occurs following intercostal nerve blockade followed in order of decreasing concentration, the lumbar epidural space, brachial plexus site, and subcutaneous tissue. The total dose injected regardless of the site is the primary determinant of the absorption rate and blood levels achieved. There is a linear relationship between the amount of lidocaine injected and the resultant peak anesthetic blood levels. Nevertheless, it has been observed that lidocaine hydrochloride is completely absorbed following parenteral administration, its rate of absorption depending also on lipid solubility and the presence or absence of a vasoconstrictor agent. Except for intravascular administration, the highest blood levels are obtained following intercostal nerve block and the lowest after subcutaneous administration. Additionally, lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Route of Elimination
The excretion of unchanged lidocaine and its metabolites occurs predominantly via the kidney with less than 5% in the unchanged form appearing in the urine. The renal clearance is inversely related to its protein binding affinity and the pH of the urine. This suggests by the latter that excretion of lidocaine occurs by non-ionic diffusion.
Volume of Distribution
The volume of distribution determined for lidocaine is 0.7 to 1.5 L/kg. In particular, lidocaine is distributed throughout the total body water. Its rate of disappearance from the blood can be described by a two or possibly even three-compartment model. There is a rapid disappearance (alpha phase) which is believed to be related to uptake by rapidly equilibrating tissues (tissues with high vascular perfusion, for example). The slower phase is related to distribution to slowly equilibrating tissues (beta phase) and to its metabolism and excretion (gamma phase). Lidocaine's distribution is ultimately throughout all body tissues. In general, the more highly perfused organs will show higher concentrations of the agent. The highest percentage of this drug will be found in skeletal muscle, mainly due to the mass of muscle rather than an affinity.
Clearance
The mean systemic clearance observed for intravenously administered lidocaine in a study of 15 adults was approximately 0.64 +/- 0.18 L/min.
Binding of lidocaine to plasma proteins is variable and concentration dependent. At concentrations of 1-4 ug/mL, the drug is approximately 60-80% bound to plasma proteins. Lidocaine is partially bound to a1-acid glycoprotein (a1-AGP), and the extent of binding to a1-AGP depends on the plasma concentration of the protein. In patients with myocardial infarction, increases in plasma a1-AGP concentration are associated with increased lidocaine binding and increased total plasma concentrations of the drug, but only small increases in plasma concentration of free drug; these changes in a1-AGP concentration and lidocaine binding are believed to account in part for accumulation of the drug observed in patients with myocardial infarction receiving prolonged infusions.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
The volume of distribution is decreased in patients with congestive heart failure and increased in patients with liver disease.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
Lidocaine is widely distributed into body tissues. After an IV bolus, there is an early, rapid decline in plasma concentrations of the drug, principally associated with distribution into highly perfused tissues such as the kidneys, lungs, liver, and heart, followed by a slower elimination phase in which metabolism and redistribution into skeletal muscle and adipose tissue occur. Lidocaine has a high affinity for fat and adipose tissue. As plasma concentrations of the drug fall, the diffusion gradient from tissue to blood increases and the lidocaine that initially entered the highly perfused tissues and fat diffuses back into the blood.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
Plasma lidocaine concentrations of approximately 1-5 ug/mL are required to suppress ventricular arrhythmias. Toxicity has been associated with plasma lidocaine concentrations greater than 5 ug/mL. Following IV administration of a bolus dose of 50-100 mg of lidocaine hydrochloride, the drug has an onset of action within 45-90 seconds and a duration of action of 10-20 minutes. If an IV infusion is begun without an initial bolus dose, the attainment of therapeutic plasma concentrations is relatively slow. For example, therapeutic plasma concentrations are achieved in 30-60 minutes after the start of a continuous infusion of 60-70 ug/kg per minute when no loading dose is given. Plasma concentrations of 1.5-5.5 ug/mL have been reported to be maintained with an initial IV bolus of 1.5 mg/kg followed by infusion of 50 ug/kg per minute in patients with heart disease.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
For more Absorption, Distribution and Excretion (Complete) data for LIDOCAINE (17 total), please visit the HSDB record page.
Lidocaine is metabolized predominantly and rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine HCl. Approximately 90% of lidocaine HCl administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline.
Approximately 90% of a parenteral dose of lidocaine is rapidly metabolized in the liver by de-ethylation to form MEGX and GX followed by cleavage of the amide bond to form xylidine and 4-hydroxyxylidine which are excreted in urine. Less than 10% of a dose is excreted unchanged in urine.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
The rate of lidocaine metabolism may also be decreased in patients with liver disease, possibly because of altered perfusion in the liver or hepatic tissue necrosis. Distribution and elimination of lidocaine and /monoethylglycinexylidide/ MEGX appear to remain normal in patients with renal failure, but /glycinexylidide/ GX may accumulate in these patients when lidocaine is administered IV for several days.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
... The purpose of this study is to determine the amount of lidocaine and its metabolite monoethyl-glycinexylidide (MEGX) in breast milk after local anesthesia during dental procedures. The study population consisted of seven nursing mothers (age, 23-39 years) who received 3.6 to 7.2 mL 2% lidocaine without adrenaline. Blood and milk concentrations of lidocaine and its metabolite MEGX were assayed using high-performance liquid chromatography. The milk-to-plasma ratio and the possible daily doses in infants for both lidocaine and MEGX were calculated. The lidocaine concentration in maternal plasma 2 hours after injection was 347.6 +/- 221.8 ug/L, the lidocaine concentration in maternal milk ranged from 120.5 +/- 54.1 ug/L (3 hours after injection) to 58.3 +/- 22.8 ug/L (6 hours after injection), the MEGX concentration in maternal plasma 2 hours after injection was 58.9 +/- 30.3 ug/L, and the MEGX concentration in maternal milk ranged from 97.5 +/- 39.6 ug/L (3 hours after injection) to 52.7 +/- 23.8 ug/L (6 hours after injection). According to these data and considering an intake of 90 mL breast milk every 3 hours, the daily infant dosages of lidocaine and MEGX were 73.41 +/- 38.94 ug/L/day and 66.1 +/- 28.5 ug/L/day respectively. This study suggests that even if a nursing mother undergoes dental treatment with local anesthesia using lidocaine without adrenaline, she can safely continue breastfeeding.
PMID:11321382 Giuliani M et al; J Pediatr Gastroenterol Nutr. 32(2):142-4. (2001).
... To determine the time/concentration profile of lidocaine and its active metabolites glycinexylidide (GX) and monoethylglycinexylidide (MEGX) during a 96 hr lidocaine infusion. lidocaine was administered to 8 mature healthy horses as a continuous rate infusion (0.05 mg/kg bwt/min) for 96 hr. Blood concentrations of lidocaine, GX and MEGX were determined using high performance liquid chromatography during and after discontinuation of the infusion. Serum lidocaine concentrations reached steady state by 3 hr and did not accumulate thereafter. Concentrations were above the target therapeutic concentration (980 ng/mL) only at 6 and 48 hr, and did not reach the range described as potentially causing toxicity (>1850 ng/mL) at any time. MEGX did not accumulate over time, while the GX accumulated significantly up to 48 hr and then remained constant. The serum concentrations of lidocaine, MEGX and GX were below the limit of detection within 24 hr of discontinuation of the infusion. None of the horses developed any signs of lidocaine toxicity during the study. The metabolism of lidocaine was not significantly impaired by prolonged infusion and no adverse effects were observed. Prolonged infusions appear to be safe in normal horses but the accumulation of GX, a potentially toxic active metabolite, is cause for concern.
PMID:18267881 Dickey EJ et al; Equine Vet J 40 (4): 348-52 (2008)
For more Metabolism/Metabolites (Complete) data for LIDOCAINE (11 total), please visit the HSDB record page.
Lidocaine has known human metabolites that include 3-Hydroxylidocaine and Monoethylglycinexylidide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life of lidocaine hydrochloride following an intravenous bolus injection is typically 1.5 to 2.0 hours. Because of the rapid rate at which lidocaine hydrochloride is metabolized, any condition that affects liver function may alter lidocaine HCl kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction.
... In 30 patients (aged 18-70 yr) undergoing surgery ... mean half-life ... lidocaine was ... 94 min.
Sanchez Alcaraz A et al; Farmacia Hosp 15 (Jul-Aug): 211-5 (1991)
... In patients with myocardial infarction (with or without cardiac failure), the half-lives of lidocaine and MEGX have been reported to be prolonged; the half-life of GX is reportedly prolonged in patients with cardiac failure secondary to myocardial infarction. The half-life of lidocaine is reportedly also prolonged in patients with congestive heart failure or liver disease and may be prolonged following continuous IV infusions lasting longer than 24 hours.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
Lidocaine has an initial half-life of 7-30 minutes and a terminal half-life of 1.5-2 hours. In healthy individuals, the elimination half-lives of the active metabolites, monoethylglycinexylidide (MEGX) and glycinexylidide (GX) are 2 hours and 10 hours, respectively...
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
Lidocaine is extensively metabolized by the liver; heaptic disease and reduced hepatic blood flow prolong the half life, which is normally < 1 hr in dogs.
National Fire Protection Association; Fire Protection Guide to Hazardous Materials. 14TH Edition, Quincy, MA 2010, p. 2162
The elimination half-life of lidocaine in the newborn following maternal epidual anesthesia averaged 3 hr.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Nineth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2011, p. 832
Lidocaine is a local anesthetic of the amide type. It is used to provide local anesthesia by nerve blockade at various sites in the body. It does so by stabilizing the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action. In particular, the lidocaine agent acts on sodium ion channels located on the internal surface of nerve cell membranes. At these channels, neutral uncharged lidocaine molecules diffuse through neural sheaths into the axoplasm where they are subsequently ionized by joining with hydrogen ions. The resultant lidocaine cations are then capable of reversibly binding the sodium channels from the inside, keeping them locked in an open state that prevents nerve depolarization. As a result, with sufficient blockage, the membrane of the postsynaptic neuron will ultimately not depolarize and will thus fail to transmit an action potential. This facilitates an anesthetic effect by not merely preventing pain signals from propagating to the brain but by aborting their generation in the first place. In addition to blocking conduction in nerve axons in the peripheral nervous system, lidocaine has important effects on the central nervous system and cardiovascular system. After absorption, lidocaine may cause stimulation of the CNS followed by depression and in the cardiovascular system, it acts primarily on the myocardium where it may produce decreases in electrical excitability, conduction rate, and force of contraction.
Abnormal, repetitive impulse firing arising from incomplete inactivation of Na+ channels may be involved in several diseases of muscle and nerve, including familial myotonias and neuropathic pain syndromes. Systemic local anesthetics have been shown to have clinical efficacy against myotonias and some forms of neuropathic pain, so we sought to develop an in vitro model to examine the cellular basis for these drugs' effects. In frog sciatic nerves, studied in vitro by the sucrose-gap method, peptide alpha-toxins from sea anemone (ATXII) or scorpion (LQIIa) venom, which inhibit Na+ channel inactivation, induced repetitively firing compound action potentials (CAPs) superimposed on a plateau depolarization lasting several seconds. The initial spike of the CAP was unaffected, but the plateau and repetitive firing were strongly suppressed by 5-30 uM lidocaine. Lidocaine caused a rapid, concentration-dependent decay of the plateau, quantitatively consistent with blockade of open Na(+) channels. Early and late repetitive firing were equally suppressed by lidocaine with IC50 = 10 uM. After washout of lidocaine and LQIIa, the plateau and repetitive firing remained for > 1 hr, showing that lidocaine had not caused dissociation of channel-bound alpha-toxin. These findings indicate that therapeutic concentrations of lidocaine can reverse the "abnormal" features of action potentials caused by non-inactivating Na+ channels without affecting the normal spike component.
PMID:11317273 Khodorova A et al; Muscle Nerve 24 (5): 634-47 (2001)
Lidocaine controls ventricular arrhythmias by suppressing automaticity in the His-Purkinje system and by suppressing spontaneous depolarization of the ventricles during diastole. These effects occur at lidocaine concentrations that do not suppress automaticity of the sinoatrial (SA) node. At therapeutic plasma concentrations, lidocaine has little effect on atrioventricular (AV) node conduction and His-Purkinje conduction in the normal heart. Specialized conducting tissues of the atria are less sensitive to the effects of lidocaine than are those of ventricular tissues. Lidocaine has a variable effect on the effective refractory period (ERP) of the AV node; the drug shortens the ERP and the action potential duration of the His-Purkinje system. Lidocaine does not appear to affect excitability of normal cardiac tissue.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 1697
Prilocaine and lidocaine are classified as amide-type local anesthetics for which serious adverse effects include methemoglobinemia. Although the hydrolyzed metabolites of prilocaine (o-toluidine) and lidocaine (2,6-xylidine) have been suspected to induce methemoglobinemia, the metabolic enzymes that are involved remain uncharacterized. In the present study, we aimed to identify the human enzymes that are responsible for prilocaine- and lidocaine-induced methemoglobinemia. Our experiments revealed that prilocaine was hydrolyzed by recombinant human carboxylesterase (CES) 1A and CES2, whereas lidocaine was hydrolyzed by only human CES1A. When the parent compounds (prilocaine and lidocaine) were incubated with human liver microsomes (HLM), methemoglobin (Met-Hb) formation was lower than when the hydrolyzed metabolites were incubated with HLM. In addition, Met-Hb formation when prilocaine and o-toluidine were incubated with HLM was higher than that when lidocaine and 2,6-xylidine were incubated with HLM. Incubation with diisopropyl fluorophosphate and bis-(4-nitrophenyl) phosphate, which are general inhibitors of CES, significantly decreased Met-Hb formation when prilocaine and lidocaine were incubated with HLM. An anti-CYP3A4 antibody further decreased the residual formation of Met-Hb. Met-Hb formation after the incubation of o-toluidine and 2,6-xylidine with HLM was only markedly decreased by incubation with an anti-CYP2E1 antibody. o-Toluidine and 2,6-xylidine were further metabolized by CYP2E1 to 4- and 6-hydroxy-o-toluidine and 4-hydroxy-2,6-xylidine, respectively, and these metabolites were shown to more efficiently induce Met-Hb formation than the parent compounds. Collectively, we found that the metabolites produced by human CES-, CYP2E1-, and CYP3A4-mediated metabolism were involved in prilocaine- and lidocaine-induced methemoglobinemia.
PMID:23530020 Higuchi R et al; Drug Metab Dispos 41 (6): 1220-30 (2013)
Lidocaine acts primarily to inhibit sodium movement across cell membranes. In peripheral nerves, this action results in a decreased rate and degree of depolarization of nerve cells and failure to achieve the threshold potential necessary to propagate action potentials, resulting in conduction blockade and anesthesia. In the heart, lidocaine also inhibits sodium conductance, decreasing the maximal rate of depolarization of myocardial conducting cells. This effect is more prominent in cells that are ischemic and at rapid heart rates. For this reason lidocaine is most effective in the termination of rapid ventricular tachycardia, especially during acute ischemia or after myocardial infarction. Lidocaine may also increase the ventricular fibrillation threshold. At therapeutic doses, lidocaine has minimal electrophysiologic effects on normal cells.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 1372