

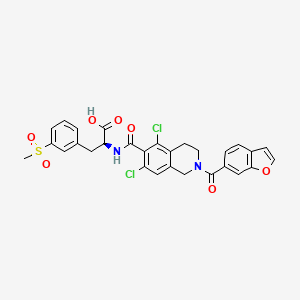

1. (2s)-2-(((2-(benzofuran-6-ylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinolin-6- Yl)carbonyl)amino)-3-(3-(methylsulfonyl)phenyl)propanoic Acid
2. L-phenylalanine, N-((2-(6-benzofuranylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl)-3-(methylsulfonyl)-
3. Lifitegrast Ophthalmic Solution
4. Sar 1118
5. Sar-1118
6. Shp-606
7. Shp606
8. Xiidra
1. 1025967-78-5
2. Xiidra
3. Sar 1118
4. Sar-1118
5. Shp606
6. Shp-606
7. (s)-2-(2-(benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxamido)-3-(3-(methylsulfonyl)phenyl)propanoic Acid
8. Chembl2048028
9. 038e5l962w
10. (2s)-2-(((2-(benzofuran-6-ylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinolin-6- Yl)carbonyl)amino)-3-(3-(methylsulfonyl)phenyl)propanoic Acid
11. L-phenylalanine, N-((2-(6-benzofuranylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl)carbonyl)-3-(methylsulfonyl)-
12. L-phenylalanine, N-[[2-(6-benzofuranylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl]carbonyl]-3-(methylsulfonyl)-
13. Lifitegrast [usan:inn]
14. Unii-038e5l962w
15. Sar1118
16. Xiidra (tn)
17. Lifitegrast [mi]
18. Lifitegrast [inn]
19. Lifitegrast (usan/inn)
20. Lifitegrast [usan]
21. Lifitegrast; Sar 1118
22. Lifitegrast [who-dd]
23. Lifitegrast Ophthalmic Solution
24. Gtpl7533
25. Schembl2632068
26. Amy4450
27. Dtxsid60145345
28. Lifitegrast [orange Book]
29. Chebi:133023
30. Ex-a2582
31. Bdbm50386331
32. Mfcd28502439
33. S3714
34. Zinc84668739
35. Ccg-270245
36. Cs-6264
37. Db11611
38. Compound 1g [pmid: 24900456]
39. Ac-32534
40. Ds-20052
41. Hy-19344
42. D10374
43. A900838
44. Q23044263
45. (2s)-2-[[2-(1-benzofuran-6-carbonyl)-5,7-dichloro3,4-dihydro-1h-isoquinoline-6-carbonyl]amino]-3-(3-methylsulfonylphenyl)propanoic Acid
46. N-[[2-(6-benzofuranylcarbonyl)-5,7-dichloro-1,2,3,4-tetrahydro-6-isoquinolinyl]carbonyl]-3-(methylsulfonyl)-l-phenylalanine
47. N-[2-(1-benzofuran-6-carbonyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carbonyl]-3-(methanesulfonyl)-l-phenylalanine
| Molecular Weight | 615.5 g/mol |
|---|---|
| Molecular Formula | C29H24Cl2N2O7S |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 614.0681277 g/mol |
| Monoisotopic Mass | 614.0681277 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of signs and symptoms of keratoconjunctivitis sicca (dry eye syndrome).
FDA Label
Treatment of dry eye disease
Lifitegrast addresses both the symptoms and the resulting ocular surface damage by interfering with ocular inflammatory cycle. Lifitegrast is a lymphocyte functionassociated antigen-1 antagonist through direct competitive antagonism and sequentially inhibits the T-cell recruitment, activation, and proinflammatory cytokine release associated with dry eye syndrome.
Ophthalmic Solutions
Sterile solutions that are intended for instillation into the eye. It does not include solutions for cleaning eyeglasses or CONTACT LENS SOLUTIONS. (See all compounds classified as Ophthalmic Solutions.)
S - Sensory organs
S01 - Ophthalmologicals
S01X - Other ophthalmologicals
S01XA - Other ophthalmologicals
S01XA25 - Lifitegrast
Absorption
The mean peak plasma concentration (Cmax) of 1.70ng/mL was reached within 15 minutes of application. Quantifiable trough plasma concentrations ranged from 0.55 ng/mL to 3.74 ng/mL. Observations show limited systemic exposure that produces significant clinical outcomes.
Route of Elimination
Not possible to perform mass balance study to determine the main route of elimination.
Clearance
Not possible to calculate clearance rate based on plasma concentrations of lifitegrast, but reported to be relatively fast in rat I.V. pharmacokinetics study. It is predicted that lifitegrast is cleared via nasal and subsequently gastrointestinal tract.
Based on the findings of an in vitro metabolism study using fresh human hepatocytes, lifitegrast does not appear to undergo significant metabolism.
Not possible to calculate plasma elimination half-life based on plasma concentrations of lifitegrast, but reported to be relatively short in rat I.V. pharmacokinetics study.
Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1). ICAM-1 may be overexpressed in corneal and conjunctival tissues in dry eye disease. LFA-1/ICAM-1 interaction can contribute to the formation of an immunological synapse resulting in T-cell proliferation/activation and migration to target tissues. In vitro studies demonstrated that lifitegrast may inhibit T-cell adhesion to ICAM-1 in a human T-cell line and may inhibit secretion of inflammatory cytokines, inflammatory mediators, chemokines, TNF-, and IL-1 in human peripheral blood mononuclear cells.
