
1. 100766, U
2. Linezolide
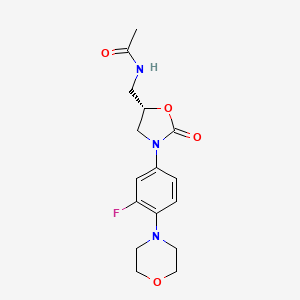
3. N-((3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide
4. Pnu 100766
5. Pnu-100766
6. Pnu100766
7. U 100766
8. U-100766
9. U100766
10. Zyvox
1. 165800-03-3
2. Zyvox
3. (s)-n-((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide
4. Pnu-100766
5. Zyvoxid
6. U-100766
7. Zyvoxam
8. Pnu 100766
9. U 100766
10. N-(((s)-3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide
11. N-[[(5s)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide
12. N-[[(5s)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide
13. Chembl126
14. Isq9i6j12j
15. U-100,766
16. Acetamide, N-[[(5s)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
17. Chebi:63607
18. Acetamide, N-((3-(3-fluoro-4-(4-morpholinyl)phenyl)-2-oxo-5-oxazolidinyl)methyl)-, (s)-
19. N-{[(5s)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl}acetamide
20. Zld
21. Zivoxid
22. Zyvoxa
23. Mfcd00937825
24. Acetamide, N-(((5s)-3-(3-fluoro-4-(4-morpholinyl)phenyl)-2-oxo-5-oxazolidinyl)methyl)-
25. N-({(5s)-3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)acetamide
26. N-[[(5s)-3-(3-fluoro-4-morpholino-phenyl)-2-oxo-oxazolidin-5-yl]methyl]acetamide
27. Smr000466335
28. Zyvox (tn)
29. Hsdb 7478
30. Sr-01000759376
31. Linezolid & Vrc3375
32. Unii-isq9i6j12j
33. Linezoid
34. Linezolid (jan/usan/inn)
35. Linezolid [usan:inn:ban]
36. (s)-n-[[3-(3-fluoro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide
37. Ncgc00164628-01
38. Linezolid (zyvox)
39. N-{[(5s)-3-[3-fluoro-4-(morpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl]methyl}acetamide
40. Pnu100766
41. 111ge017
42. Cpd000466335
43. Nda 21-130 Zyvox (linezolid Tablets)
44. Linezolid [inn]
45. Linezolid [jan]
46. Linezolid [mi]
47. Linezolid [hsdb]
48. Linezolid [usan]
49. Linezolid [vandf]
50. Linezolid [mart.]
51. (s)-n-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide
52. Linezolid [usp-rs]
53. Linezolid [who-dd]
54. Schembl5027
55. Dsstox_cid_26489
56. Dsstox_rid_81660
57. Dsstox_gsid_46489
58. Mls000759444
59. Mls001424075
60. Bidd:gt0404
61. Benzotriazol-2-yl-acetonitrile
62. Cid_441401
63. Linezolid (pnu-100766)
64. Linezolid [orange Book]
65. Dtxsid5046489
66. Linezolid, >=98% (hplc)
67. Nda 21-131 Zyvox For Injection (linezolid Injection)
68. Gtpl10827
69. Linezolid [usp Monograph]
70. Hms2051f08
71. Hms2089k06
72. Hms3260c14
73. Hms3713k10
74. Nda 21-132 Zyvox Oral Suspension (linzolid Oral Suspension)
75. Bcp05586
76. Zinc2008866
77. Tox21_112246
78. Tox21_500096
79. Bdbm50116067
80. S1408
81. Akos016340522
82. Am84567
83. Bcp9000855
84. Ccg-101009
85. Cs-0756
86. Db00601
87. Ks-1178
88. Nc00259
89. Linezolid 100 Microg/ml In Acetonitrile
90. Ncgc00260781-01
91. Ncgc00263531-03
92. Ncgc00263531-05
93. Ncgc00263531-10
94. Hy-10394
95. Cas-165800-03-3
96. L0362
97. Sw197639-3
98. C08146
99. D00947
100. Ab00639994-06
101. Ab00639994-08
102. Ab00639994-09
103. Ab00639994_10
104. Ab00639994_11
105. 800l033
106. A810662
107. Q411377
108. Q-201308
109. Sr-01000759376-4
110. Sr-01000759376-5
111. Sr-01000759376-11
112. Linezolid, United States Pharmacopeia (usp) Reference Standard
113. (s)-n-((3-fluoro-4-morpholinophenyl)-2-oxaoxazolidin-5-yl)methyl)acetamide
114. Linezolid, Pharmaceutical Secondary Standard; Certified Reference Material
115. (linezolid)n-[3-(3-fluoro-4-morpholin-4-yl-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide
116. (s)-n-((3-(3-fluoro-4-(4-morpholinyl)phenyl)-2-oxo-5-oxazolidinyl)methyl)acetamide
117. (s)-n-[ [3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-oxazolidin-5-yl]methyl]-acetamide
118. (s)-n-[[3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-acetamide
119. N-[(r)-3-(3-fluoro-4-morpholin-4-yl-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide
120. N-[(s)-3-(3-fluoro-4-morpholin-4-yl-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide
121. N-[3-(3-fluoro-4-morpholin-4-yl-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide
122. N-[3-(3-fluoro-4-morpholin-4-yl-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide(linezolid)
123. (s)-3-(3-fluoro-4-morpholin-4-yl-phenyl)-5-[(1-hydroxy-ethylamino)-methyl]-oxazolidin-2-one
124. N-((s)-2-oxo-3-(s)-2,3,3a,4-tetrahydro-1h-benzo[b]pyrrolo[1,2-d][1,4]oxazin-7-yl-oxazolidin-5-ylmethyl)-acetamide
125. N-[[(5s)-3-(3-fluoranyl-4-morpholin-4-yl-phenyl)-2-oxidanylidene-1,3-oxazolidin-5-yl]methyl]ethanamide
| Molecular Weight | 337.35 g/mol |
|---|---|
| Molecular Formula | C16H20FN3O4 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 337.14378429 g/mol |
| Monoisotopic Mass | 337.14378429 g/mol |
| Topological Polar Surface Area | 71.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 472 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Linezolid |
| PubMed Health | Linezolid |
| Drug Classes | Antibiotic |
| Drug Label | ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazoli... |
| Active Ingredient | Linezolid; Linezolid sodium chloride |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | oral; Injection; Oral |
| Strength | 200mg/100ml; 600mg/300ml; 100mg/5ml; 600mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharma; Hospira; Sandoz; Roxane; Glenmark Generics; Teva Pharms; Gate Pharma |
| 2 of 4 | |
|---|---|
| Drug Name | Zyvox |
| PubMed Health | Linezolid |
| Drug Classes | Antibiotic |
| Drug Label | ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazoli... |
| Active Ingredient | Linezolid |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Injection; Oral |
| Strength | 200mg/100ml; 100mg/5ml; 600mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 3 of 4 | |
|---|---|
| Drug Name | Linezolid |
| PubMed Health | Linezolid |
| Drug Classes | Antibiotic |
| Drug Label | ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazoli... |
| Active Ingredient | Linezolid; Linezolid sodium chloride |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | oral; Injection; Oral |
| Strength | 200mg/100ml; 600mg/300ml; 100mg/5ml; 600mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharma; Hospira; Sandoz; Roxane; Glenmark Generics; Teva Pharms; Gate Pharma |
| 4 of 4 | |
|---|---|
| Drug Name | Zyvox |
| PubMed Health | Linezolid |
| Drug Classes | Antibiotic |
| Drug Label | ZYVOX I.V. Injection, ZYVOX Tablets, and ZYVOX for Oral Suspension contain linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazoli... |
| Active Ingredient | Linezolid |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Injection; Oral |
| Strength | 200mg/100ml; 100mg/5ml; 600mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 986
Intravenous and oral linezolid is indicated in the treatment of nosocomial pneumonia caused by methicillin-susceptible and methicillin resistant Staphylococcus aureus or penicillin-susceptible strains of Streptococcus pneumonia. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
Intravenous and oral linezolid is indicated in the treatment of vancomycin-resistant Enterococcus faecium infections. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
Oral linezolid is indicated in the treatment of uncomplicated skin and soft tissue infections caused by methicillin-susceptible strains of Staphylococcus aureus or Streptococcus pyogenes. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
For more Therapeutic Uses (Complete) data for LINEZOLID (16 total), please visit the HSDB record page.
Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia) has been reported in patients receiving linezolid. In cases where the outcome is known, when linezolid was discontinued, the affected hematologic parameters have risen toward pretreatment levels. Complete blood counts should be monitored weekly in patients who receive linezolid, particularly in those who receive linezolid for longer than two weeks, those with pre-existing myelosuppression, those receiving concomitant drugs that produce bone marrow suppression, or those with a chronic infection who have received previous or concomitant antibiotic therapy. Discontinuation of therapy with linezolid should be considered in patients who develop or have worsening myelosuppression.
Prescribing Information for Zyvox (Linezolid); Pharmacia & Upjohn, Div. of Pfizer Inc, USA; 2006. Available from, as of October 22, 2006: https://www.zyvox.com/mediaPi.asp
Lactic acidosis has been reported with the use of Zyvox. In reported cases, patients experienced repeated episodes of nausea and vomiting. Patients who develop recurrent nausea or vomiting, unexplained acidosis, or a low bicarbonate level while receiving zyvox should receive immediate medical evaluation.
Prescribing Information for Zyvox (Linezolid); Pharmacia & Upjohn, Div. of Pfizer Inc, USA; 2006. Available from, as of October 22, 2006: https://www.zyvox.com/mediaPi.asp
Spontaneous reports of serotonin syndrome associated with the co-administration of Zyvox and serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs), have been reported. Where administration of Zyvox and concomitant serotonergic agents is clinically appropriate, patients should be closely observed for signs and symptoms of serotonin syndrome such as cognitive dysfunction, hyperpyrexia, hyperreflexia and incoordination. If signs or symptoms occur physicians should consider discontinuation of either one or both agents.
Prescribing Information for Zyvox (Linezolid); Pharmacia & Upjohn, Div. of Pfizer Inc, USA; 2006. Available from, as of October 22, 2006: https://www.zyvox.com/mediaPi.asp
Peripheral and optic neuropathy have been reported in patients treated with Zyvox, primarily those patients treated for longer than the maximum recommended duration of 28 days. In cases of optic neuropathy that progressed to loss of vision, patients were treated for extended periods beyond the maximum recommended duration. Visual blurring has been reported in some patients treated with Zyvox for less than 28 days. If patients experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, blurred vision, or visual field defect, prompt ophthalmic evaluation is recommended. Visual function should be monitored in all patients taking Zyvox for extended periods (> or = 3 months) and in all patients reporting new visual symptoms regardless of length of therapy with Zyvox. If peripheral or optic neuropathy occurs, the continued use of Zyvox in these patients should be weighed against the potential risks.
Prescribing Information for Zyvox (Linezolid); Pharmacia & Upjohn, Div. of Pfizer Inc, USA; 2006. Available from, as of October 22, 2006: https://www.zyvox.com/mediaPi.asp
For more Drug Warnings (Complete) data for LINEZOLID (18 total), please visit the HSDB record page.
Linezolid is indicated in adults and children for the treatment of infections caused by susceptible Gram-positive bacteria, including nosocomial pneumonia, community-acquired pneumonia, skin and skin structure infections, and vancomycin-resistant _Enterococcus faecium_ infections. Examples of susceptible bacteria include _Staphylococcus aureus_, _Streptococcus pneumoniae_, _Streptococcus pyogenes_, and _Streptococcus agalactiae_. Linezolid is not indicated for the treatment of Gram-negative infections, nor has it been evaluated for use longer than 28 days.
FDA Label
Linezolid is an oxazolidinone antibacterial agent effective against most strains of aerobic Gram-positive bacteria and mycobacteria. It appears to be bacteriostatic against both staphylococci and enterococci and bactericidal against most isolates of streptococci. Linezolid has shown some _in vitro_ activity against Gram-negative and anaerobic bacteria but is not considered efficacious against these organisms. Linezolid is a reversible and non-selective inhibitor of monoamine oxidase (MAO) enzymes and can therefore contribute to the development of serotonin syndrome when administered alongside serotonergic agents such as selective serotonin re-uptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs). Linezolid should not be used for the treatment of catheter-related bloodstream infections or catheter-site infections, as the risk of therapy appears to outweigh its benefits under these circumstances.
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XX08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XX - Other antibacterials
J01XX08 - Linezolid
Absorption
Linezolid is extensively absorbed following oral administration and has an absolute bioavailability of approximately 100%. Maximum plasma concentrations are reached within approximately 1 to 2 hours after dosing (Tmax) and range from 8.1-12.9 mcg/mL after single doses and 11.0-21.2 mcg/mL after multiple dosing. The absorption of orally administered linezolid is not significantly affected by co-administration with food and it may therefore be given without regard to the timing of meals.
Route of Elimination
Urinary excretion is the primary means by which linezolid and its metabolic products are excreted. Following the administration of a radiolabeled dose of linezolid under steady-state conditions, approximately 84% of radioactivity was recovered in the urine, of which approximately 30% is unchanged parent drug, 40% is the hydroxyethyl glycine metabolite, and 10% is the aminoethoxyacetic acid metabolite. Fecal elimination is comparatively minor, with no parent drug observed in feces and only 6% and 3% of an administered dose found in the feces as the hydroxyethyl glycine metabolite and the aminoethoxyacetic acid metabolite, respectively.
Volume of Distribution
At steady-state, the volume of distribution of linezolid in healthy adults is approximately 40-50 liters.
Clearance
Total clearance of linezolid is estimated to be 100-200 mL/min, the majority of which appears to be non-renal. Mean renal clearance is approximately 40 mL/min, which suggests net tubular reabsorption, while non-renal clearance is estimated to account for roughly 65% of total clearance, or 70-150 mL/min on average. Variability in linezolid clearance is high, particularly for non-renal clearance.
Distributed to well-perfused tissues; volume of distribution slightly lower in women than men. VolD (steady state) - 40 to 50 L.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
AUC is lower for pediatric patients compared with adults and a wider variability of linezolid AUC cross all pediatric age groups as compared with adults. Most pre-term neonates less than 7 days of age (gestational age less than 34 weeks) have larger AUC values than many full-term neonates and older infants.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
Linezolid was rapidly absorbed after p.o. dosing with an p.o. bioavailability of > 95% in rat and dog, and > 70% in mouse. Twenty-eight-day i.v./p.o. toxicokinetic studies in rat (20-200 mg kg(-1) day(-1)) and dog (10-80 mg kg(-1) day(-1)) revealed neither a meaningful increase in clearance nor accumulation upon multiple dosing. Linezolid had limited protein binding (<35%) and was very well distributed to most extravascular sites, with a volume of distribution at steady-state (V(ss)) approximately equal to total body water. Linezolid circulated mainly as parent drug and was excreted mainly as parent drug and two inactive carboxylic acids, PNU-142586 and PNU-142300. Minor secondary metabolites were also characterized. In all species, the clearance rate was determined by metabolism. Radioactivity recovery was essentially complete within 24-48 hr. Renal excretion of parent drug and metabolites was a major elimination route. Parent drug underwent renal tubular reabsorption, significantly slowing parent drug excretion and allowing a slow metabolic process to become rate-limiting in overall clearance. It is concluded that ADME data were relatively consistent across species and supported the rat and dog as the principal non-clinical safety species.
PMID:12419019 Slatter JG et al; Xenobiotica 32 (10): 907-24 (2002)
In two randomized, double-blind, placebo-controlled, dose-escalating trials, subjects were exposed either to oral (375, 500 or 625 mg) or intravenous (500 or 625 mg) linezolid or placebo twice daily. Serial blood and urine samples were obtained after the first- and multiple-dose administrations for up to 18 days. Non-compartmental pharmacokinetic analyses were used to describe the disposition of linezolid. Plasma linezolid concentrations and area under the concentration-time curves (AUC) increased proportionally with dose irrespective of the route of administration. Plasma linezolid concentrations remained above the MIC90 for susceptible target pathogens (4.0 mg/L) for the majority of the 12 hr dosing interval. Mean clearance, half-life and volume of distribution were similar irrespective of dose for both the oral and intravenous routes. Linezolid was well tolerated and the frequency of drug-related adverse events was similar between the linezolid and placebo groups. Oral and intravenous linezolid exhibit linear pharmacokinetics, with concentrations remaining above the target MIC90 /minimal inhibitory concentration/ for most of the dosing interval. These results support a twice-daily schedule for linezolid and demonstrate the feasibility of converting from intravenous to oral dosing without a dose adjustment.
PMID:12668582 Stalker DJ et al; J Antimicrob Chemother 51 (5): 1239-46 (2003)
For more Absorption, Distribution and Excretion (Complete) data for LINEZOLID (16 total), please visit the HSDB record page.
Linezolid is primarily metabolized to two inactive metabolites: an aminoethoxyacetic acid metabolite (PNU-142300) and a hydroxyethyl glycine metabolite (PNU-142586), both of which are the result of morpholine ring oxidation. The hydroxyethyl glycine metabolite - the most abundant of the two metabolites - is likely generated via non-enzymatic processes, though further detail has not been elucidated. While the specific enzymes responsible for the biotransformation of linezolid are unclear, it does not appear to be subject to metabolism via the CYP450 enzyme system, nor does it meaningfully inhibit or induce these enzymes. Linezolid is, however, a reversible and non-selective inhibitor of monoamine oxidase enzymes.
In vitro studies have not shown that linezolid is metabolized by human cytochrome p450 enzymes. Linezolid does not inhibit the cytochrome p450 enzymes.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
Linezolid is primarily metabolized via oxidation of the morpholine ring. Two inactive metabolites are formed: the aminoethoxyacetic acid metabolite and the hydroxyethyl glycine metabolite. The hydroxyethyl glycine metabolite is formed via a non-enzymatic chemical oxidation mechanism in vitro.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
The drug is metabolized principally via oxidation to 2 inactive metabolites; an aminoethoxyacetic acid metabolite and a hydroxyethyl glycine metabolite. Linezolid is not metabolized to any measurable extent by the cytochrome p450 (CYP) enzyme system. Linezolid does not inhibit CYP isoenzymes 1A2, 2C9, 2C19, 2D6, 2E1, or 3A4 and is not an enzyme inducer, suggesting that the drug is unlikely to alter the pharmacokinetics of drugs metabolized by these enzymes.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 492
In vitro studies were conducted to identify the hepatic enzyme(s) responsible for the oxidative metabolism of linezolid. In human liver microsomes, linezolid was oxidized to a single metabolite, hydroxylinezolid (M1). Formation of M1 was determined to be dependent upon microsomal protein and NADPH. Over a concentration range of 2 to 700 uM, the rate of M1 formation conformed to first-order (nonsaturable) kinetics. Application of conventional in vitro techniques were unable to identify the molecular origin of M1 based on the following experiments: a) inhibitor/substrates for various cytochrome P-450 (CYP) enzymes were unable to inhibit M1 formation; b) formation of M1 did not correlate (r(2) < 0.23) with any of the measured catalytic activities across a population of human livers (n = 14); c) M1 formation was not detectable in incubations using microsomes prepared from a baculovirus insect cell line expressing CYPs 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, 3A5, and 4A11. In addition, results obtained from an in vitro P-450 inhibition screen revealed that linezolid was devoid of any inhibitory activity toward the following CYP enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). Additional in vitro studies excluded the possibility of flavin-containing monooxygenase and monoamine oxidase as potential enzymes responsible for metabolite formation. However, metabolite formation was found to be optimal under basic (pH 9.0) conditions, which suggests the potential involvement of either an uncharacterized P-450 enzyme or an alternative microsomal mediated oxidative pathway.
PMID:10950842 Wynalda MA et al; Drug Metab Dispos 28 (9): 1014-7 (2000)
Linezolid is primarily metabolized by oxidation of the morpholine ring, which results in two inactive ring-opened carboxylic acid metabolites: the aminoethoxyacetic acid metabolite (A), and the hydroxyethyl glycine metabolite (B). Formation of metabolite B is mediated by a non-enzymatic chemical oxidation mechanism in vitro. Linezolid is not an inducer of cytochrome P450 (CYP) in rats, and it has been demonstrated from in vitro studies that linezolid is not detectably metabolized by human cytochrome P450 and it does not inhibit the activities of clinically significant human CYP isoforms (1A2, 2C9, 2C19, 2D6, 2E1, 3A4).
Prescribing Information for Zyvox (Linezolid); Pharmacia & Upjohn, Div. of Pfizer Inc, USA; 2006. Available from, as of October 22, 2006: https://www.zyvox.com/mediaPi.asp
The elimination half-life is estimated to be between 5 and 7 hours.
... A significant although weak correlation between age and total body clearance was observed. The mean (+ or - SD) values for elimination half-life, total clearance and apparent volume of distribution were 3.0 + or - 1.1 hr, 0.34 + or - 0.15 liter/h/kg and 0.73 + or - 0.18 liter/kg, respectively. ...
PMID:11144380 Kearns GL et al; Pediatr Infect Dis J 19 (12): 1178-84 (2000)
The following are elimination half live values of linezolid doses in adults: 400 mg tablet (single dose) - 5.2 hours; 400 mg tablet every 12 hours - 4.69 hours; 600 mg tablet (single dose) - 4.26 hours; 600 mg tablet every 12 hours - 5.4 hours; 600 mg oral suspension (single dose) - 4.6 hours; 600 mg intravenous injection (single dose) - 4.4 hours; 600 mg intravenous injection every 12 hours - 4.8 hours;. Pediatrics ranging in age from greater than 7 days of age to 11 years of age have a shorter half-life compared with adults.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1944
Linezolid exerts its antibacterial effects by interfering with bacterial protein translation. It binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, which is essential for bacterial reproduction, thereby preventing bacteria from dividing. Point mutations in the bacterial 23S rRNA can lead to linezolid resistance, and the development of linezolid-resistant _Enterococcus faecium_ and _Staphylococcus aureus_ have been documented during its clinical use. As antimicrobial susceptibility patterns are geographically distinct, local antibiograms should be consulted to ensure adequate coverage of relevant pathogens prior to use.
Linezolid is a synthetic oxazolidinone anti-infective agent that is structurally unrelated to other anti-infectives commercially available in the US. In contrast to other anti-infectives that inhibit bacterial protein synthesis, linezolid acts early in translation by binding to a site on the bacterial 23S ribosomal RNA of the 50S subunit and preventing the formation of a functional 70S initiation complex, which is an essential component of the bacterial translation process.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 492
Linezolid acts via inhibition of protein synthesis. It bind to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex. This step is essential for the bacterial translation process.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 1943
Linezolid is an oxazolidinone antibiotic that is increasingly used to treat drug-resistant, gram-positive pathogens. The mechanism of action is inhibition of bacterial protein synthesis. Optic and/or peripheral neuropathy and lactic acidosis are reported side effects, but the underlying pathophysiological mechanism has not been unravelled. Mitochondrial ultrastructure, mitochondrial respiratory chain enzyme activity /were studied/, and mitochondrial DNA (mtDNA) in muscle, liver, and kidney samples obtained from a patient who developed optic neuropathy, encephalopathy, skeletal myopathy, lactic acidosis, and renal failure after prolonged use of linezolid. In addition, mtDNA, respiratory chain enzyme activity, and protein amount in muscle and liver samples obtained from experimental animals that received linezolid or placebo /were evaluated/. In the patient, mitochondrial respiratory chain enzyme activity was decreased in affected tissues, without ultrastructural mitochondrial abnormalities and without mutations or depletion of mtDNA. In the experimental animals, linezolid induced a dose- and time-dependent decrease of the activity of respiratory chain complexes containing mtDNA-encoded subunits and a decreased amount of protein of these complexes, whereas the amount of mtDNA was normal. These results provide direct evidence that linezolid inhibits mitochondrial protein synthesis with potentially severe clinical consequences.
PMID:16575728 De Vriese AS et al Clin Infect Dis 42 (8): 1111-7 (2006)