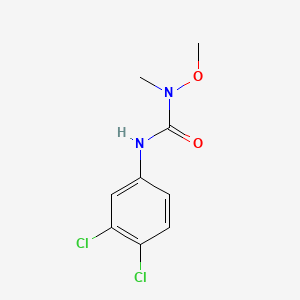

1. Afalon
1. 330-55-2
2. 3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea
3. Methoxydiuron
4. Afalon
5. Cephalon
6. Aphalon
7. Linurex
8. Garnitan
9. Lorox
10. Herbicide 326
11. Afalon Inuron
12. Linorox
13. Rotalin
14. Sarclex
15. Sinuron
16. Du Pont 326
17. Linex 4l
18. Laroks
19. N'-(3,4-dichlorophenyl)-n-methoxy-n-methylurea
20. Dupont Herbicide 326
21. Urea, N'-(3,4-dichlorophenyl)-n-methoxy-n-methyl-
22. Lorox Linuron Weed Killer
23. 1-methoxy-1-methyl-3-(3,4-dichlorophenyl)urea
24. Hoe 2810
25. Urea, 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl-
26. 3-(3,4-dichlorophenyl)-1-methoxy-1-methyl-urea
27. N-(3,4-dichlorophenyl)-n'-methyl-n'-methoxyurea
28. 01xp1su59o
29. Chebi:6482
30. Linex
31. 1-(3,4-dichlorophenyl)3-methoxy-3-methyluree
32. 3-(3,4-dichloor-fenyl)-1-methoxy-1-methylureum
33. 3-(3,4-dichloro-fenil)-1-metossi-1-metil-urea
34. 3-(4,5-dichlorphenyl)-1-methoxy-1-methylharnstoff
35. N-(3,4-dwuchlorofenylo)n'-metoksy-n'-metylomocznik
36. Linuron 10 Microg/ml In Acetonitrile
37. 3-(3,4-dichlor-phenyl)-1-methoxy-1-methyl-harnstoff
38. Linuron 100 Microg/ml In Acetonitrile
39. Malurane
40. Profalon
41. Soilcid
42. Certroli-lin
43. Dsstox_cid_4163
44. Laroks [polish]
45. Du Pont Herbicide 326
46. Dsstox_rid_77311
47. Lorox Weed Killer
48. Dsstox_gsid_24163
49. Linuron (herbicide)
50. Caswell No. 528
51. Alfalon
52. Linuron Solution
53. Linuron [ansi:bsi:iso]
54. Cas-330-55-2
55. Linuron [iso]
56. Hsdb 1733
57. Hoe 002810
58. Hoe 02 810
59. Einecs 206-356-5
60. Epa Pesticide Chemical Code 035506
61. Brn 2128725
62. Unii-01xp1su59o
63. Norunil
64. Scarclex
65. Atlas Linuron
66. 1-(3,4-dichlorophenyl)3-methoxy-3-methyluree [french]
67. 3-(3,4-dichloor-fenyl)-1-methoxy-1-methylureum [dutch]
68. 3-(3,4-dicloro-fenil)-1-metossi-1-metil-urea [italian]
69. 3-(3,4-dichloro-fenil)-1-metossi-1-metil-urea [italian]
70. 3-(4,5-dichlorphenyl)-1-methoxy-1-methylharnstoff [german]
71. 3-(3,4-dichlor-phenyl)-1-methoxy-1-methyl-harnstoff [german]
72. Linuron [hsdb]
73. N-(3,4-dwuchlorofenylo)n'-metoksy-n'-metylomocznik [polish]
74. Linuron [mi]
75. 3-(3,4-dicloro-fenil)-1-metossi-1-metil-urea
76. Schembl56566
77. N-(3,4-dichlorophenyl)-n'-methoxy-n'-methylurea
78. Bidd:er0455
79. Chembl448213
80. Dtxsid2024163
81. Xkjmbincvninca-uhfffaoysa-
82. Zinc900693
83. Hy-b1866
84. Linuron 100 Microg/ml In Methanol
85. Tox21_201281
86. Tox21_300732
87. Linuron 100 Microg/ml In N-hexane
88. Stk664290
89. Akos015889915
90. Ncgc00160429-01
91. Ncgc00160429-02
92. Ncgc00160429-03
93. Ncgc00160429-04
94. Ncgc00160429-05
95. Ncgc00160429-06
96. Ncgc00254638-01
97. Ncgc00258833-01
98. Db-048328
99. Linuron, Pestanal(r), Analytical Standard
100. Cs-0013933
101. Ft-0603379
102. L0395
103. 330l552
104. A821586
105. N'-(3,4-dichlorophenyl)-n-methoxy-n-methyl Urea
106. Q421476
107. J-018993
108. Linuron Solution, 100 Mug/ml In Methanol, Pestanal(r), Analytical Standard
| Molecular Weight | 249.09 g/mol |
|---|---|
| Molecular Formula | C9H10Cl2N2O2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 248.0119330 g/mol |
| Monoisotopic Mass | 248.0119330 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 228 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
IN CHRONIC TOXICITY STUDIES ... LINURON ... CONTINUOUSLY FED TO RATS & DOGS FOR ... TWO YR AT DIETARY LEVELS ... FROM 25 TO 2500 PPM ... TOTAL ANILINE-CONTAINING RESIDUES IN BLOOD & ... (MUSCLE, FAT, LIVER, KIDNEY, SPLEEN) WERE A FEW TO 100 PPM. ... RESIDUES REPRESENTED ONLY MINUTE FRACTION OF ... HERBICIDE INGESTED BY ANIMALS.
Kearney, P.C., and D. D. Kaufman (eds.) Herbicides: Chemistry, Degredation and Mode of Action. Volumes 1 and 2. 2nd ed. New York: Marcel Dekker, Inc., 1975., p. 237
LINURON IS MOST READILY ABSORBED THROUGH THE ROOT SYSTEM; LESS SO THROUGH FOLIAGE & STEMS. HOWEVER, FOLIAR ABSORPTION ... IS SIGNIFICANTLY GREATER THAN THAT OF DIURON, MONURON, OR FENURON. ... TRANSLOCATION IS PRIMARILY UPWARD IN THE XYLEM.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 287
... RESIDUE DATA ... OBTAINED IN SHORT TERM ELIMINATION EXPERIMENTS ... . AMT OFRADIOACTIVITY WHICH PERSISTED IN BLOOD & DIFFERENT TISSUES AT END OF 72 HR ... PERIODS WERE NO MORE & USUALLY MUCH LESS THAN 1% OF DOSE APPLIED.
Kearney, P.C., and D. D. Kaufman (eds.) Herbicides: Chemistry, Degredation and Mode of Action. Volumes 1 and 2. 2nd ed. New York: Marcel Dekker, Inc., 1975., p. 237
... LINURON-INDUCIBLE ENZYME WAS OBTAINED FROM BACILLUS SPHAERICUS. THIS ACYLAMIDASE DEGRADED LINURON BY HYDROLYSIS OF AMIDE BOND WITH ... RELEASE OF CARBON DIOXIDE & N,O-DIMETHYL HYDROXYLAMINE. ... ENZYME WAS SPECIFIC FOR METHOXY-SUBSTITUTED PHENYLUREAS & DID NOT HYDROLYZE 1,1-DIMETHYL PHENYLUREAS ... .
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 382
IN GREENHOUSE STUDIES, LINURON ENTERED CORN (ZEA MAYS L), SOYBEAN (GLYCINE MAX L) & CRABGRASS (DIGITARIA SANGUINALIS L) WITH ABSORBED WATER. DEMETHYL LINURON & 3,4-DICHLORO-ANILINE WERE FOUND IN TISSUES. ... SOME LINURON BOUND WITHIN PLANT ... .
Menzie, C. M. Metabolism of Pesticides, An Update. U.S. Department of the Interior, Fish, Wild-life Service, Special Scientific Report - Wildlife No. 184, Washington, DC: U.S. Government Printing Office, l974., p. 382
LINURON ... FED TO ALBINO RATS. URINE ... ANALYZED FOR METABOLITES /&/ N-(3,4-DICHLOROPHENYL)UREA, N-(3,4-DICHLOROPHENYL)-N'-METHYLUREA & 3,4-DICHLOROANILINE WERE FOUND FREE. N-(2-HYDROXY-4,5-DICHLOROPHENYL)-N'-METHYLUREA, N-(5-HYDROXY-3,4-DICHLOROPHENYL)UREA & 6-ACETAMIDO-2,3-DICHLOROPHENOL ... /WERE IDENTIFIED/ AS GLUCURONIDES OR SULFATES.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 340
In rats linuron is metabolized by demethoxylation followed by hydroxylation of the benzene ring. Major urinary metabolites are urea derivatives; no unchanged linuron could be demonstrated. Only trace amounts of 3,4-dichloroaniline were found. If metabolism to dichloroaniline is major pathway in humans, ... methemoglobinemia should be anticipated after toxic doses.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-331
For more Metabolism/Metabolites (Complete) data for LINURON (9 total), please visit the HSDB record page.
Inhibits photosynthesis.
Hartley, D. and H. Kidd (eds.). The Agrochemicals Handbook. 2nd ed. Lechworth, Herts, England: The Royal Society of Chemistry, 1987., p. A248/Aug 87