
1. 2211, Nn
2. Nn 2211
3. Nn-2211
4. Nn2211
5. Saxenda
6. Victoza
1. Victoza
2. 204656-20-2
3. Liraglutida
4. Liraglutidum
5. Nn2211
6. Nn-2211
7. Nn 2211
8. Chembl4084119
9. Saxenda
10. Liraglutidum [inn-latin]
11. Liraglutida [inn-spanish]
12. Unii-839i73s42a
13. Hsdb 8205
14. Liraglutide [usan:inn:ban:jan]
15. Gtpl1133
16. Chebi:71193
17. Dtxsid60174433
18. Ex-a2418
19. Bdbm50240819
20. Akos037435224
21. N26-(hexadecanoyl-gamma-glutamyle)-(34-arginine)glp-1-(7-37)-peptide
22. 839i73s42a
23. N26-(hexadecanoyl-gamma-glutamyle)-(34-arginine)glucagon-like-peptide-1-(7-37)-peptide
24. As-56276
25. A16115
26. Arg34lys26-(n-epsilon-(gamma-glu(n-alpha-hexadecanoyl)))-glp-1(7-37)
27. N(sup 26)-(hexadecanoyl-gamma-glutamyle)-(34-arginine)glp-1-(7-37)-peptide
| Molecular Weight | 3751 g/mol |
|---|---|
| Molecular Formula | C172H265N43O51 |
| XLogP3 | -3.4 |
| Hydrogen Bond Donor Count | 54 |
| Hydrogen Bond Acceptor Count | 55 |
| Rotatable Bond Count | 132 |
| Exact Mass | 3749.9498161 g/mol |
| Monoisotopic Mass | 3748.9464612 g/mol |
| Topological Polar Surface Area | 1510 Ų |
| Heavy Atom Count | 266 |
| Formal Charge | 0 |
| Complexity | 8760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 31 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Victoza |
| PubMed Health | Liraglutide (Injection) |
| Drug Classes | Antidiabetic |
| Drug Label | Victoza contains liraglutide, an analog of human GLP-1 and acts as a GLP-1 receptor agonist. The peptide precursor of liraglutide, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae, has been engineered to b... |
| Active Ingredient | Liraglutide recombinant |
| Dosage Form | Solution |
| Route | Subcutaneous |
| Strength | 18mg/3ml (6mg/ml) |
| Market Status | Prescription |
| Company | Novo Nordisk |
| 2 of 2 | |
|---|---|
| Drug Name | Victoza |
| PubMed Health | Liraglutide (Injection) |
| Drug Classes | Antidiabetic |
| Drug Label | Victoza contains liraglutide, an analog of human GLP-1 and acts as a GLP-1 receptor agonist. The peptide precursor of liraglutide, produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae, has been engineered to b... |
| Active Ingredient | Liraglutide recombinant |
| Dosage Form | Solution |
| Route | Subcutaneous |
| Strength | 18mg/3ml (6mg/ml) |
| Market Status | Prescription |
| Company | Novo Nordisk |
Hypoglycemic Agents
National Library of Medicine's Medical Subject Headings. Liraglutide. Online file (MeSH, 2014). Available from, as of April 30 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Victoza is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. /Included in US product label/
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Because of the uncertain relevance of the rodent thyroid C-cell tumor findings to humans, prescribe Victoza only to patients for whom the potential benefits are considered to outweigh the potential risk. Victoza is not recommended as first-line therapy for patients who have inadequate glycemic control on diet and exercise. ... Victoza is not a substitute for insulin. Victoza should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
EXPL THER: According to World Health Organization estimates, type 2 diabetes (T2D) is an epidemic (particularly in under developed countries) and a socio-economic challenge. This is even more relevant since increasing evidence points to T2D as a risk factor for Alzheimer's disease (AD), supporting the hypothesis that AD is a "type 3 diabetes" or "brain insulin resistant state". Despite the limited knowledge on the molecular mechanisms and the etiological complexity of both pathologies, evidence suggests that neurodegeneration/death underlying cognitive dysfunction (and ultimately dementia) upon long-term T2D may arise from a complex interplay between T2D and brain aging. Additionally, decreased brain insulin levels/signaling and glucose metabolism in both pathologies further suggests that an effective treatment strategy for one disorder may be also beneficial in the other. In this regard, one such promising strategy is a novel successful anti-T2D class of drugs, the glucagon-like peptide-1 (GLP-1) mimetics (e.g. exendin-4 or liraglutide), whose potential neuroprotective effects have been increasingly shown in the last years. In fact, several studies showed that, besides improving peripheral (and probably brain) insulin signaling, GLP-1 analogs minimize cell loss and possibly rescue cognitive decline in models of AD, Parkinson's (PD) or Huntington's disease. Interestingly, exendin-4 is undergoing clinical trials to test its potential as an anti-PD therapy. Herewith, we aim to integrate the available data on the metabolic and neuroprotective effects of GLP-1 mimetics in the central nervous system (CNS) with the complex crosstalk between T2D-AD, as well as their potential therapeutic value against T2D-associated cognitive dysfunction. C
PMID:23314196 Duarte AI et al; Biochim Biophys Acta 1832 (4): 527-41 (2013)
/BOXED WARNING/ WARNING: RISK OF THYROID C-CELL TUMORS. Liraglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures in both genders of rats and mice. It is unknown whether Victoza causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as human relevance could not be ruled out by clinical or nonclinical studies. Victoza is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Based on the findings in rodents, monitoring with serum calcitonin or thyroid ultrasound was performed during clinical trials, but this may have increased the number of unnecessary thyroid surgeries. It is unknown whether monitoring with serum calcitonin or thyroid ultrasound will mitigate human risk of thyroid C-cell tumors. Patients should be counseled regarding the risk and symptoms of thyroid tumors.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
There have been postmarketing reports of serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema) in patients treated with Victoza. If a hypersensitivity reaction occurs, the patient should discontinue Victoza and other suspect medications and promptly seek medical advice.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Based on spontaneous postmarketing reports, acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with Victoza. After initiation of Victoza, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, Victoza should promptly be discontinued and appropriate management should be initiated. If pancreatitis is confirmed, Victoza should not be restarted. Consider antidiabetic therapies other than Victoza in patients with a history of pancreatitis.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
In postmarketing reports, acute renal failure and worsening of chronic renal failure (which may require hemodialysis) have been reported with liraglutide. Some of these events occurred in patients without known underlying renal disease. Most of these events occurred in patients experiencing nausea, vomiting, diarrhea, or dehydration. Some of these events occurred in patients receiving liraglutide in combination with one or more agents known to affect renal function or hydration status. Liraglutide has not been found to be directly nephrotoxic in preclinical or clinical studies. Renal effects usually have been reversible with supportive treatment and discontinuance of potentially causative agents, including liraglutide. Clinicians should use caution when initiating liraglutide or escalating dosage in patients with renal impairment.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3217
For more Drug Warnings (Complete) data for Liraglutide (15 total), please visit the HSDB record page.
Liraglutide is indicated in combination with diet and exercise to improve glycemic control in patients 10 years and older with type 2 diabetes mellitus. It is also indicated to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes mellitus as well as cardiovascular disease.
FDA Label
Saxenda is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients with an initial Body Mass Index (BMI) of
30 kg/m (obese), or
27 kg/m to < 30 kg/m (overweight) in the presence of at least one weight-related comorbidity such as dysglycaemia (pre-diabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia or obstructive sleep apnoea.
Treatment with Saxenda should be discontinued after 12 weeks on the 3. 0 mg/day dose if patients have not lost at least 5% of their initial body weight.
Victoza is indicated for the treatment of adults, adolescents and children aged 10 years and above with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise
- as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
- in addition to other medicinal products for the treatment of diabetes.
For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied.
Treatment of type II diabetes mellitus
Treatment of obesity
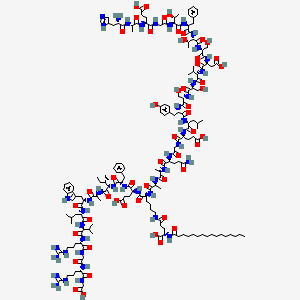
Liraglutide is a once-daily GLP-1 derivative for the treatment of type 2 diabetes. The prolonged action of liraglutide is achieved by attaching a fatty acid molecule at position 26 of the GLP-1 molecule, enabling it to bind reversibly to albumin within the subcutaneous tissue and bloodstream and be released slowly over time. Binding with albumin results in slower degradation and reduced elimination of liraglutide from the circulation by the kidneys compared to GLP-1. The effect of liraglutide is the increased secretion of insulin and decreased secretion of glucagon in response to glucose as well as slower gastric emptying. Liraglutide also does not adversely affect glucagon secretion in response to low blood sugar.
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Incretins
Peptides which stimulate INSULIN release from the PANCREATIC BETA CELLS following oral nutrient ingestion, or postprandially. (See all compounds classified as Incretins.)
A10BJ02
A10BJ02
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BJ - Glucagon-like peptide-1 (glp-1) analogues
A10BJ02 - Liraglutide
Absorption
Bioavailability of liraglutide after subcutaneous injection is approximately 55% and maximum concentrations are reached after 11.7 hours.
Route of Elimination
6% excreted in urine and 5% excreted in feces.
Volume of Distribution
13L.
Clearance
1.2L/h.
The mean apparent volume of distribution after subcutaneous administration of Victoza 0.6 mg is approximately 13 L. The mean volume of distribution after intravenous administration of Victoza is 0.07 L/kg. Liraglutide is extensively bound to plasma protein (>98%).
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Following a 3(H)-liraglutide dose, intact liraglutide was not detected in urine or feces. Only a minor part of the administered radioactivity was excreted as liraglutide-related metabolites in urine or feces (6% and 5%, respectively). The majority of urine and feces radioactivity was excreted during the first 6-8 days. The mean apparent clearance following subcutaneous administration of a single dose of liraglutide is approximately 1.2 L/hr with an elimination half-life of approximately 13 hours, making Victoza suitable for once daily administration.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Following subcutaneous administration, maximum concentrations of liraglutide are achieved at 8-12 hours post dosing. The mean peak (Cmax) and total (AUC) exposures of liraglutide were 35 ng/mL and 960 ng hr/mL, respectively, for a subcutaneous single dose of 0.6 mg. After subcutaneous single dose administrations, Cmax and AUC of liraglutide increased proportionally over the therapeutic dose range of 0.6 mg to 1.8 mg. At 1.8 mg Victoza, the average steady state concentration of liraglutide over 24 hours was approximately 128 ng/mL. AUC0-8 was equivalent between upper arm and abdomen, and between upper arm and thigh. AUC0-8 from thigh was 22% lower than that from abdomen. However, liraglutide exposures were considered comparable among these three subcutaneous injection sites. Absolute bioavailability of liraglutide following subcutaneous administration is approximately 55%.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Liraglutide is a novel once-daily human glucagon-like peptide (GLP)-1 analog in clinical use for the treatment of type 2 diabetes. To study metabolism and excretion of 3(H)-liraglutide, a single subcutaneous dose of 0.75 mg/14.2 MBq was given to healthy males. The recovered radioactivity in blood, urine, and feces was measured, and metabolites were profiled. In addition, 3(H)-liraglutide and [(3)H]GLP-1(7-37) were incubated in vitro with dipeptidyl peptidase-IV (DPP-IV) and neutral endopeptidase (NEP) to compare the metabolite profiles and characterize the degradation products of liraglutide. The exposure of radioactivity in plasma (area under the concentration-time curve from 2 to 24 hr) was represented by liraglutide (> or = 89%) and two minor metabolites (totaling < or =11%). Similarly to GLP-1, liraglutide was cleaved in vitro by DPP-IV in the Ala8-Glu9 position of the N terminus and degraded by NEP into several metabolites. The chromatographic retention time of DPP-IV-truncated liraglutide correlated well with the primary human plasma metabolite [GLP-1(9-37)], and some of the NEP degradation products eluted very close to both plasma metabolites. Three minor metabolites totaling 6 and 5% of the administered radioactivity were excreted in urine and feces, respectively, but no liraglutide was detected. In conclusion, liraglutide is metabolized in vitro by DPP-IV and NEP in a manner similar to that of native GLP-1, although at a much slower rate. The metabolite profiles suggest that both DPP-IV and NEP are also involved in the in vivo degradation of liraglutide. The lack of intact liraglutide excreted in urine and feces and the low levels of metabolites in plasma indicate that liraglutide is completely degraded within the body.
PMID:20709939 Malm-Erjefalt M et al; Drug Metab Dispos 38 (11): 1944-53 (2010)
For more Absorption, Distribution and Excretion (Complete) data for Liraglutide (8 total), please visit the HSDB record page.
Liraglutide is less sensitive to metabolism than the endogenous GLP-1 and so is more slowly metabolized by dipeptidyl peptidase-4 and neutral endopeptidase to various smaller polypeptides which have not all been structurally determined. A portion of Liraglutide may be completely metabolized to carbon dioxide and water.
The metabolic and excretion patterns were highly similar across species with liraglutide being fully metabolised in the body by sequential cleavage of small peptide fragments and amino acids. The in vitro metabolism studies indicate that the initial metabolism involves cleavage of the peptide backbone with no degradation of the glutamate-palmitic acid side-chain. Mice, rats and monkeys displayed similar plasma profiles and showed no significant gender differences. A higher number of metabolites were observed in plasma from the animal species (especially the rat and monkey) as compared to human plasma. This disparity can partly be explained by differences in the sample preparation as human plasma samples were freeze dried prior to analysis causing a removal of volatile metabolites (including tritiated water). All detected metabolites were minor and obtained in low amount (<15%) and therefore no structural identification of these was performed. This is acceptable since the metabolites are only formed in low amounts and since the metabolites are expected to resemble endogenous substances with well-known metabolic pathways
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Victoza (Liraglutide) p.10 (2009). Available from, as of July 16, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001026/WC500050016.pdf
During the initial 24 hours following administration of a single 3(H)-liraglutide dose to healthy subjects, the major component in plasma was intact liraglutide. Liraglutide is endogenously metabolized /SRP: in a manner similar to large proteins/ without a specific organ as a major route of elimination.
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Terminal half life of 13 hours.
The terminal half-life of liraglutide seems to be similar in pigs (approximately 14 hr) and humans (approximately 15 hr) while shorter in mice, rats, rabbits and monkeys (4-8 hr). Several studies in monkeys, pigs and humans indicated that extravascular administration (SC and pulmonary) of liraglutide prolongs the terminal half-life as compared to intravenous (IV) administration. Furthermore, the terminal half-life seemed also to be prolonged by repeated dosing in rats, monkeys, pigs and humans. This tendency was not apparent for mice and rabbits.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Victoza (Liraglutide) p.9 (2009). Available from, as of July 16, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001026/WC500050016.pdf
elimination half-life ... approximately 13 hours
NIH; DailyMed. Current Medication Information for Victoza (Liraglutide (rDNA Origin) Injection) Injection, Solution (Revised: April 2013). Available from, as of July 15, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4
Liraglutide is an acylated synthetic glucagon-like peptide-1 analog. Liraglutide is an agonist of the glucagon-like peptide-1 receptor which is coupled to adenylate cyclase. The increase in cyclic AMP stimulates the glucose dependant release of insulin, inhibits the glucose dependant release of glucagon, and slows gastric emptying to increase control of blood sugar.
Liraglutide is an acylated, long-acting, human glucagon-like peptide-1 (GLP-1) receptor agonist; the synthetic (recombinant DNA origin) peptide precursor of liraglutide has 97% amino acid sequence homology to endogenous human GLP-1-(7-37). Liraglutide is prepared by attaching palmitic acid with a glutamic acid spacer on the lysine residue at position 26 of the peptide precursor. GLP-1-(7-37) represents less than 20% of total circulating endogenous GLP-1. Like GLP-1-(7-37), liraglutide activates the GLP-1 receptor in pancreatic beta cells. Liraglutide also increases intracellular cyclic 3',5'-adenosine monophosphate (cAMP) leading to insulin release in the presence of elevated glucose concentrations. This insulin secretion subsides as blood glucose concentrations decrease and approach euglycemia. In addition, liraglutide suppresses glucagon secretion in a glucose-dependent manner but does not impair normal glucagon response to hypoglycemia. Liraglutide delays gastric emptying, reducing the rate at which postprandial glucose appears in the circulation. As a result of these actions resulting in increased insulin secretion, suppression of glucagon secretion, and delays in gastric emptying, liraglutide effectively reduces fasting and postprandial plasma glucose concentrations in patients with type 2 diabetes mellitus.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 3217-8
Liraglutide is a glucagon-like peptide-1 (GLP-1) mimetic used for the treatment of Type 2 diabetes. Similar to the actions of endogenous GLP-1, liraglutide potentiates the post-prandial release of insulin, inhibits glucagon release and increases satiety. Recent epidemiological studies and clinical trials have suggested that treatment with GLP-1 mimetics may also diminish the risk of cardiovascular disease in diabetic patients. The mechanism responsible for this effect has yet to be determined; however, one possibility is that they might do so by a direct effect on vascular endothelium. Since low grade inflammation of the endothelium is an early event in the pathogenesis of atherosclerotic cardiovascular disease (ASCVD), we determined the effects of liraglutide on inflammation in cultured human aortic endothelial cells (HAECs). Liraglutide reduced the inflammatory responses to TNFalpha and LPS stimulation, as evidenced by both reduced protein expression of the adhesion molecules VCAM-1 and E-Selectin, and THP-1 monocyte adhesion. This was found to result from increased cell Ca2+ and several molecules sensitive to Ca2+ with known anti inflammatory actions in endothelial cells, including CaMKKbeta, CaMKI, AMPK, eNOS and CREB. Treatment of the cells with STO-609, a CaMKK inhibitor, diminished both the activation of AMPK, CaMKI and the inhibition of TNFa and LPS-induced monocyte adhesion by liraglutide. Likewise, expression of an shRNA against AMPK nullified the anti-inflammatory effects of liraglutide. The results indicate that liraglutide exerts a strong anti-inflammatory effect on HAECs. They also demonstrate that this is due to its ability to increase intracellular Ca2+ and activate CAMKKbeta, which in turn activates AMPK.
PMID:2483525 Krasner NM et al; PLoS One. 2014 May 16;9(5):e97554. doi: 10.1371/journal.pone.0097554. eCollection 2014.
In vivo, liraglutide lowers blood glucose and body weight in a number of diabetic and obese models using rodents, pigs and monkeys. The mechanism of action in vivo involved glucose-dependent increase in insulin secretion, lowered glucagon secretion, decreased gastric emptying, loss of body fat, lowered food intake, altered food preference, and maintained energy expenditure. The mechanism of action is consistent with a specific GLP-1 effect.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Victoza (Liraglutide) p.9 (2009). Available from, as of July 16, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001026/WC500050016.pdf
Liraglutide is a long-acting GLP-1 analogue, designed to bind to albumin as the main molecular mechanism of protraction. In vitro, this was shown in the receptor cAMP as well as binding assay where addition of albumin right-shifted the dose-response and/or binding curve. The apparent reduced potency of liraglutide underlines that only the free fraction of liraglutide is responsible for its pharmacological effect in vitro as well as in vivo. Furthermore, liraglutide in a pharmaceutical solution forms a micell-like heptamer which may contribute to the slow absorption from the subcutis.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Victoza (Liraglutide) p.8 (2009). Available from, as of July 16, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001026/WC500050016.pdf