
1. Apo Primidone
2. Apo-primidone
3. Desoxyphenobarbital
4. Liskantin
5. Misodine
6. Mizodin
7. Mylepsinum
8. Mysoline
9. Primaclone
10. Primidon Holsten
11. Resimatil
12. Sertan
1. 125-33-7
2. Primaclone
3. Mysoline
4. Lepimidin
5. 2-deoxyphenobarbital
6. Liskantin
7. Mylepsinum
8. Lepsiral
9. Misodine
10. Mizodin
11. Mylepsin
12. Neurosyn
13. Primidon
14. Sertan
15. 2-desoxyphenobarbital
16. Hexadiona
17. Majsolin
18. Milepsin
19. Misolyne
20. Prilepsin
21. Primakton
22. Primoline
23. Prysoline
24. Resimatil
25. Midone
26. Mizolin
27. Mysedon
28. Cyral
29. Medi-pets
30. Primacone
31. Desoxyphenobarbitone
32. Pyrimidone Medi-pets
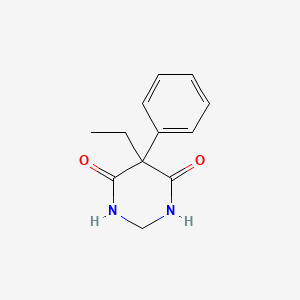
33. 5-ethyl-5-phenyl-1,3-diazinane-4,6-dione
34. Primidona
35. Primidonum
36. Roe 101
37. 5-phenyl-5-ethyl-hexahydropyrimidine-4,6-dione
38. 4,6(1h,5h)-pyrimidinedione, 5-ethyldihydro-5-phenyl-
39. 5-ethyl-5-phenyldihydropyrimidine-4,6(1h,5h)-dione
40. Nci-c56360
41. Primidone (primaclone)
42. 5-ethyldihydro-5-phenyl-4,6(1h,5h)-pyrimidinedione
43. 5-ethyl-5-phenylhexahydropyrimidine-4,6-dione
44. 5-ethylhexahydro-4,6-dioxo-5-phenylpyrimidine
45. 5-ethylhexahydro-5-phenylpyrimidine-4,6-dione
46. Nsc 41701
47. 5-aethyl-5-phenyl-hexahydropyrimidin-4,6-dion
48. Chebi:8412
49. 5-phenyl-5-aethylhexahydropyrimidindion-(4,6)
50. Nsc-41701
51. Mls000028593
52. Primacione
53. 13afd7670q
54. Ncgc00015834-11
55. Cas-125-33-7
56. Smr000058501
57. Dsstox_cid_3510
58. Primidonum [inn-latin]
59. Dsstox_rid_77058
60. Dsstox_gsid_23510
61. Primidona [inn-spanish]
62. Hexamidine (the Antispasmodic)
63. Ccris 54
64. Mysoline (tn)
65. Hsdb 3169
66. Sr-01000003162
67. Einecs 204-737-0
68. Brn 0218034
69. Hexamidine(the Antispasmodic)
70. 5-aethyl-5-phenyl-hexahydropyrimidin-4,6-dion [german]
71. 5-phenyl-5-aethylhexahydropyrimidindion-(4,6) [german]
72. Unii-13afd7670q
73. Primidone [usp:inn:ban:jan]
74. Primidone Solution
75. Primidone(mysoline)
76. Primidone (mysoline)
77. Spectrum_000832
78. Tocris-0830
79. Pyrimidone 'medi-pets'
80. Primidone [inn]
81. Primidone [jan]
82. Primidone [mi]
83. Primidone [hsdb]
84. Primidone [iarc]
85. Opera_id_1083
86. Prestwick0_000933
87. Prestwick1_000933
88. Prestwick2_000933
89. Prestwick3_000933
90. Spectrum2_001293
91. Spectrum3_000553
92. Spectrum4_000485
93. Spectrum5_001144
94. Lopac-p-7295
95. Primidone [vandf]
96. Biomol-nt_000261
97. Chembl856
98. Primidone [mart.]
99. P 7295
100. Pyrimidone ''medi-pets''
101. Primidone [usp-rs]
102. Primidone [who-dd]
103. Lopac0_001021
104. Schembl34221
105. Bspbio_000866
106. Bspbio_002225
107. Kbiogr_000969
108. Kbioss_001312
109. 5-24-08-00102 (beilstein Handbook Reference)
110. Mls001055411
111. Mls001074125
112. Bidd:gt0319
113. Divk1c_000324
114. Primidone, Analytical Standard
115. Spectrum1500501
116. Spbio_001325
117. Spbio_003035
118. Primidone (jp17/usp/inn)
119. Primidone [green Book]
120. Bpbio1_000859
121. Bpbio1_000954
122. Gtpl5338
123. Zinc1979
124. Primidone [ep Impurity]
125. Primidone [orange Book]
126. Dtxsid7023510
127. Primidone [ep Monograph]
128. Hms501a06
129. Kbio1_000324
130. Kbio2_001312
131. Kbio2_003880
132. Kbio2_006448
133. Kbio3_001725
134. Primidone For Peak Identification
135. Wln: T6mv Dvmtj C2 Cr
136. Primidone [usp Monograph]
137. Ninds_000324
138. Hms1570l08
139. Hms1920j20
140. Hms2092a21
141. Hms2097l08
142. Hms2236g03
143. Hms3259k04
144. Hms3263m03
145. Hms3266h18
146. Hms3369j12
147. Hms3411n13
148. Hms3655l05
149. Hms3675n13
150. Hms3714l08
151. Hms3884a18
152. Pharmakon1600-01500501
153. Hy-b0339
154. Nsc41701
155. Tox21_110238
156. Tox21_201948
157. Tox21_303194
158. Tox21_501021
159. Bdbm50248152
160. Ccg-39231
161. Mfcd00038662
162. Nsc757291
163. S1965
164. Akos003368409
165. Tox21_110238_1
166. Db00794
167. Ks-5217
168. Lp01021
169. Nc00532
170. Nsc-757291
171. Sdccgsbi-0050994.p005
172. Idi1_000324
173. Smp1_000135
174. Ncgc00015834-01
175. Ncgc00015834-02
176. Ncgc00015834-03
177. Ncgc00015834-04
178. Ncgc00015834-05
179. Ncgc00015834-06
180. Ncgc00015834-07
181. Ncgc00015834-08
182. Ncgc00015834-09
183. Ncgc00015834-10
184. Ncgc00015834-12
185. Ncgc00015834-13
186. Ncgc00015834-14
187. Ncgc00015834-15
188. Ncgc00015834-16
189. Ncgc00015834-17
190. Ncgc00015834-19
191. Ncgc00015834-28
192. Ncgc00016377-01
193. Ncgc00023254-02
194. Ncgc00023254-04
195. Ncgc00023254-05
196. Ncgc00023254-06
197. Ncgc00023254-07
198. Ncgc00023254-08
199. Ncgc00023254-09
200. Ncgc00023254-10
201. Ncgc00257146-01
202. Ncgc00259497-01
203. Ncgc00261706-01
204. Bp166193
205. Sbi-0050994.p004
206. Ab00052079
207. Eu-0101021
208. Ft-0603328
209. Ft-0674032
210. P1906
211. Sw196576-3
212. 5-ethyldihydro-5-phenyl-4,5h)-pyrimidinedione
213. C07371
214. C71997
215. D00474
216. Ab00052079-14
217. Ab00052079_15
218. Ab00052079_16
219. 125p337
220. 4,5h)-pyrimidinedione, 5-ethyldihydro-5-phenyl-
221. A805374
222. Q420383
223. J-005215
224. Sr-01000003162-2
225. Sr-01000003162-4
226. Sr-01000003162-5
227. Sr-01000003162-8
228. Brd-k32247306-001-05-4
229. Brd-k32247306-001-17-9
230. 5-ethyl-5-phenyldihydro-4,6(1h,5h)-pyrimidinedione #
231. Primidone, European Pharmacopoeia (ep) Reference Standard
232. Primidone, United States Pharmacopeia (usp) Reference Standard
233. Primidone For Peak Identification, European Pharmacopoeia (ep) Reference Standard
234. Primidone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 218.25 g/mol |
|---|---|
| Molecular Formula | C12H14N2O2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 218.105527694 g/mol |
| Monoisotopic Mass | 218.105527694 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 279 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Mysoline |
| PubMed Health | Primidone (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula:Mysoline* (primidone) is a white, crystalline, highly stable substance, M.P. 279-284C. It is poorly soluble in water (60 mg per 100 mL at 37C) and in most org... |
| Active Ingredient | Primidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 50mg |
| Market Status | Prescription |
| Company | Valeant |
| 2 of 4 | |
|---|---|
| Drug Name | Primidone |
| PubMed Health | Primidone (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Primidone, USP is a white, crystalline, highly stable substance, M.P. 279-284 C. It is poorly soluble in water (60 mg per 100 mL at 37 C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog.Chemi... |
| Active Ingredient | Primidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 50mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Hikma Pharms; Watson Labs; Amneal Pharm; Lannett; Mutual Pharm; Impax Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Mysoline |
| PubMed Health | Primidone (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula:Mysoline* (primidone) is a white, crystalline, highly stable substance, M.P. 279-284C. It is poorly soluble in water (60 mg per 100 mL at 37C) and in most org... |
| Active Ingredient | Primidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 50mg |
| Market Status | Prescription |
| Company | Valeant |
| 4 of 4 | |
|---|---|
| Drug Name | Primidone |
| PubMed Health | Primidone (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Primidone, USP is a white, crystalline, highly stable substance, M.P. 279-284 C. It is poorly soluble in water (60 mg per 100 mL at 37 C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog.Chemi... |
| Active Ingredient | Primidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 50mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Hikma Pharms; Watson Labs; Amneal Pharm; Lannett; Mutual Pharm; Impax Labs |
Anticonvulsants
National Library of Medicine's Medical Subject Headings. Primidone. Online file (MeSH, 2018). Available from, as of August 29, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Primidone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 29, 2018: https://clinicaltrials.gov/
Primidone, used alone or concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy. /Included in US product labeling./
NIH; DailyMed. Current Medication Information for Primidone Tablet (Updated: July 26, 2018). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1a817f7-c190-4825-94eb-442f477187e3
/EXPL THER/ OBJECTIVES: Cerebellar tremor is a disabling sign of multiple sclerosis (MS), and various kinds of treatments have been proposed with different results. Primidone is one of the medications, mostly advised for essential tremor. The aim of our study was to determine the tolerability and efficacy of primidone in reducing severe cerebellar tremor in patients with MS. METHODS: Ten patients with severe cerebellar tremor were enrolled in this study. Primidone started with dose of 31.5 mg and gradually increased up to maximum of 750 mg/d. The severity of tremor was assessed with Activity of Daily Living (ADL), Nine-Hole Peg Test (NHPT), and Fahn Tremor Rating Scale (FTRS) at baseline and 2 follow-up studies after 6 and 12 weeks. RESULTS: All outcome measures including ADL, FTRS, and NHPT of dominant and nondominant hands improved. The mean ADL changed from 51.8 at baseline to 36.8 after 12 weeks. FTRS was 14.8 at baseline, which reduced to 9.5 during this period. These changes were statistically significant. Although the time of the NHPT showed some improvement, it did not reach a statistically significant point after 6 weeks.The drug was well tolerated in all patients, and mild drowsiness reported by the patients disappeared at the end of the study. CONCLUSIONS: Our study showed that primidone is tolerable in MS patients and effectively reduces severe cerebellar tremor in such patients.
PMID:22821064 Naderi F et al; Clin Neuropharmacol 35 (5): 224-6 (2012)
For more Therapeutic Uses (Complete) data for Primidone (6 total), please visit the HSDB record page.
Primidone is contraindicated in: 1) patients with porphyria and 2) patients who are hypersensitive to phenobarbital
NIH; DailyMed. Current Medication Information for Primidone Tablet (Updated: July 26, 2018). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1a817f7-c190-4825-94eb-442f477187e3
The abrupt withdrawal of antiepileptic medication may precipitate status epilepticus. The therapeutic efficacy of a dosage regimen takes several weeks before it can be assessed.
NIH; DailyMed. Current Medication Information for Primidone Tablet (Updated: July 26, 2018). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1a817f7-c190-4825-94eb-442f477187e3
Antiepileptic drugs (AEDs), including primidone, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
NIH; DailyMed. Current Medication Information for Primidone Tablet (Updated: July 26, 2018). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1a817f7-c190-4825-94eb-442f477187e3
The most frequently occurring early side effects are ataxia and vertigo. These tend to disappear with continued therapy, or with reduction of initial dosage. Occasionally, the following have been reported: nausea, anorexia, vomiting, fatigue, hyperirritability, emotional disturbances, sexual impotency, diplopia, nystagmus, drowsiness, and morbilliform skin eruptions. Granulocytopenia, agranulocytosis, and red-cell hypoplasia and aplasia, have been reported rarely. These and, occasionally, other persistent or severe side effects may necessitate withdrawal of the drug. Megaloblastic anemia may occur as a rare idiosyncrasy to primidone and to other anticonvulsants. The anemia responds to folic acid without necessity of discontinuing medication.
NIH; DailyMed. Current Medication Information for Primidone Tablet (Updated: July 26, 2018). Available from, as of November 27, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1a817f7-c190-4825-94eb-442f477187e3
For more Drug Warnings (Complete) data for Primidone (12 total), please visit the HSDB record page.
Primidone is commonly indicated for the management of grand mal, psychomotor, and focal epileptic seizures. In addition, it has also been studied and utilized as an effective management of essential tremor.
FDA Label
Primidone alters sodium and calcium channel transport, reducing the frequency of nerve firing, which may be responsible for its effect on convulsions and essential tremor. Primidone has a wide therapeutic window as doses of 50-1000mg/day were effective. Patients should be counselled regarding the risk of status epilepticus with abrupt cessation of primidone.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AA - Barbiturates and derivatives
N03AA03 - Primidone
Absorption
Oral primidone is up to 80% bioavailable with a Tmax if 2-4h. A 500mg oral dose of primidone Reaches a Cmax of 2.70.4g/mL with a Tmax of 0.5-7h. Data regarding the AUC of primidone is not readily available.
Route of Elimination
Primidone is 72.9-80.6% recovered in urine.
Volume of Distribution
The volume of distribution of primidone is 0.5-0.8L/kg.
Clearance
Primidone is cleared at a rate of 30mL/min.
Mice were treated with a teratogenic dose of primidone (100 mg/kg) by gastric intubation at three different times during pregnancy, viz. days 6-14, days 12-14 on day 14 only. Blood samples were taken on day 14 at 1, 4, 8 and 24 hr after dosage. Primidone and its metabolites phenylethylmalondiamide (PEMA), and phenobarbitone, were assayed by GLC. There was no accumulation of the parent compound or the metabolites after repeated administration of primidone; each of the substances was cleared from the plasma within 24 hr. The rate of metabolism of primidone increased with prolonged treatment. The peak concentration of the metabolites was higher in the two multiple-dose groups than in the single dose group. The concentration of PEMA exceeded that of primidone between 3-8 hr and then began to decrease in the multiple-dose groups, a similar pattern was established for phenobarbitone also, although the concentrations were lower than those of PEMA.
PMID:910462 McElhatton PR et al; Xenobiotica 7 (10): 617-22 (1977)
The placental transfer of primidone and metabolites was investigated in 14 women treated for epilepsy with primidone (and additionally phenytoin, ethosuximide or valproate in 5 women) throughout pregnancy. Primidone, PEMA /phenylethylmalonamide/, phenobarbital, and polar metabolites (p-hydroxyphenobarbital and p-hydroxyphenobarbital glucuronide) were found in similar concentrations in maternal and cord blood at birth.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 196 (2016)
The pharmacokinetics of primidone (PRM) after oral administration of a single 500 mg dose was studied in 7 patients with acute viral hepatitis and 7 healthy control subjects. The elimination half-life and the apparent clearance of unchanged PRM in the patients were 18.0 +/- 3.1 hr and 42 +/- 14 mL/kg/hr, respectively (mean +/- SD) and did not differ significantly from the values in the controls (half-life 17.0 +/- 2.4 hr; clearance 35 +/- 8 mL/kg/hr). The metabolite phenylethylmalonamide (PEMA) was detected in the serum of all normal subjects within 2-24 hr. By contrast, serum levels of this metabolite were undetectable (less than 2 umol/L) in all but one of the patients. Serum levels of phenobarbital (PB) remained below the limit of detection (less than 2 umol/L) in all subjects. The findings indicate that accumulation of PRM with its attendant toxicity is unlikely to occur in epileptic patients who develop acute viral hepatitis, despite evidence that the metabolism of the drug is affected by this condition. The possibility of impaired conversion to PB and its implications are discussed.
PMID:6519155 Pisani F et al; Eur J Clin Pharmacol 27 (4): 465-9 (1984)
Primidone is slowly absorbed after oral administration in the dog, with peak levels occurring 2-4 hours after dosing.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 1212
For more Absorption, Distribution and Excretion (Complete) data for Primidone (11 total), please visit the HSDB record page.
Primidone is metabolized to phenobarbitol and phenylethylmalonamide (PEMA). This metabolism is largely mediated by CYP2C9, CYP2C19, and CYP2E1.
Physiologically based pharmacokinetic modeling of the parent chemical primidone and its two metabolites phenobarbital and phenylethylmalonamide (PEMA) was applied to investigate the differences of primidone metabolism among humans, rats, and mice. The model simulated previously published pharmacokinetic data of the parent chemical and its metabolites in plasma and brain tissues from separate studies of the three species. Metabolism of primidone and its metabolites varied widely among a sample of three human subjects from two separate studies. Estimated primidone metabolism, as expressed by the maximal velocity Vmax, ranged from 0 to 0.24 mg/kg/min for the production of phenobarbital and from 0.003 to 0.02 mg/kg/min for the production of PEMA among three human subjects. Further model simulations indicated that rats were more efficient at producing and clearing phenobarbital and PEMA than mice. However, the overall metabolism profile of primidone and its metabolites in mice indicated that mice were at higher risk of toxicity owing to higher residence of phenobarbital in their tissues and owing to the carcinogenic potential of phenobarbital as illustrated in long-term bioassays. ...
PMID:9616196 El-Masri HA, Portier CJ; Drug Metab Dispos 26 (6): 585-94 (1998)
The placental transfer of primidone and metabolites was investigated in 14 women treated for epilepsy with primidone (and additionally phenytoin, ethosuximide or valproate in 5 women) throughout pregnancy. Primidone, PEMA /phenylethylmalonamide/, phenobarbital, and polar metabolites (p-hydroxyphenobarbital and p-hydroxyphenobarbital glucuronide) were found in similar concentrations in maternal and cord blood at birth.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 196 (2016)
The pharmacokinetics and metabolism of primidone at steady-state were studied in 10 elderly patients aged 70-81 years and eight control subjects aged 18-26 years. Primidone half-lives and clearance values (mean +/- s.d.) were similar in the elderly and in the young (12.1 +/- 4.6 vs 14.7 +/- 3.5 hr and 34.8 +/- 9.0 vs 33.2 +/- 7.2 mL/kg/hr respectively. The serum concentrations of the metabolites phenylethylmalonamide (PEMA) and phenobarbitone relative to those of parent drug were higher in the elderly than in the young, the difference being significant (P less than 0.01) in the case of PEMA. The renal clearances of primidone, phenobarbitone and PEMA were moderately decreased in the elderly but this reduction was statistically significant only for PEMA. Elderly patients excreted a reduced proportion of unchanged primidone and an increased proportion of PEMA in urine. Ageing is associated with a greater accumulation of PEMA, which is unlikely to have a major clinical significance.
PMID:2291873 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1368252 Martines C et al; Br J Clin Pharmacol 30 (4): 607-11 (1990)
Although phenobarbital was not detected after administration of single doses of primidone, long-term administration of primidone (at various doses) in 46 epilepsy patients showed serum accumulation of phenobarbital, and PEMA /phenylethylmalonamide/. Although there was significant inter-individual variability, concentrations of the two metabolites showed correlation with those of the parent drug, and concentrations of phenobarbital were consistently higher than those of PEMA. Two of the subjects had been on a daily dose of primidone (750 mg in divided doses) for more than 3 years. After a single dose of 750 mg in this study, peak serum concentrations of primidone were achieved rapidly (by 0.5 hour), and declined slowly (half-lives, 5.3 and 7.0 hours). In both subjects, peak concentrations of metabolites, PEMA (12 and 10 ug/mL) and phenobarbital (33 and 11 ug/mL), remained relatively constant. In the cerebrospinal fluid, binding to protein by PEMA and by primidone was negligible, and approximately 60% by phenobarbital.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 195 (2016)
For more Metabolism/Metabolites (Complete) data for Primidone (9 total), please visit the HSDB record page.
The half life of primidone is 7-22h in adults, 5-11h in children, and 8-80h in newborns.
The pharmacokinetics and metabolism of primidone at steady-state were studied in 10 elderly patients aged 70-81 years and eight control subjects aged 18-26 years. Primidone half-lives and clearance values (mean +/- s.d.) were similar in the elderly and in the young (12.1 +/- 4.6 vs 14.7 +/- 3.5 hr and 34.8 +/- 9.0 vs 33.2 +/- 7.2 mL/kg/hr respectively. ...
PMID:2291873 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1368252 Martines C et al; Br J Clin Pharmacol 30 (4): 607-11 (1990)
In a ... study in eight epileptic patients (aged 18-26 years) receiving long-term treatment with primidone (mean daily dose, 422 +/- 115 mg per day), the half-life for primidone was 14.7 +/- 3.5 hours.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V108 195 (2016)
The pharmacokinetics of primidone (PRM) after oral administration of a single 500 mg dose was studied in 7 patients with acute viral hepatitis and 7 healthy control subjects. The elimination half-life and the apparent clearance of unchanged PRM in the patients were 18.0 +/- 3.1 hr and 42 +/- 14 mL/kg/hr, respectively (mean +/- SD) and did not differ significantly from the values in the controls (half-life 17.0 +/- 2.4 hr; clearance 35 +/- 8 mL/kg/hr). ...
PMID:6519155 Pisani F et al; Eur J Clin Pharmacol 27 (4): 465-9 (1984)
Serum half lives of primidone, PEMA /phenylethamalonamide/, and phenobarbital have been reported to be 1.85 hr, 7.1 hr, and 41 hr, respectively.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 1212
For more Biological Half-Life (Complete) data for Primidone (6 total), please visit the HSDB record page.
Primidone and its metabolites, phenobarbital and phenylethylmalonamide (PEMA), are active anticonvulsants. Primidone does not directly interact with GABA-A receptors or chloride channels but phenobarbital does. Primidone alters transmembrane sodium and calcium channel transport, reducing the frequency of nerve firing, which may be responsible for the primidones effect on convulsions and essential tremor.
The melastatin-related transient receptor potential (TRP) channel TRPM3 is a nonselective cation channel expressed in nociceptive neurons and activated by heat. Because TRPM3-deficient mice show inflammatory thermal hyperalgesia, pharmacological inhibition of TRPM3 may exert antinociceptive properties. Fluorometric Ca influx assays and a compound library containing approved or clinically tested drugs were used to identify TRPM3 inhibitors. Biophysical properties of channel inhibition were assessed using electrophysiological methods. The nonsteroidal anti-inflammatory drug diclofenac, the tetracyclic antidepressant maprotiline, and the anticonvulsant primidone were identified as highly efficient TRPM3 blockers with half-maximal inhibition at 0.6 to 6 uM and marked specificity for TRPM3. Most prominently, primidone was biologically active to suppress TRPM3 activation by pregnenolone sulfate (PregS) and heat at concentrations markedly lower than plasma concentrations commonly used in antiepileptic therapy. Primidone blocked PregS-induced Cai influx through TRPM3 by allosteric modulation and reversibly inhibited atypical inwardly rectifying TRPM3 currents induced by coapplication of PregS and clotrimazole. In vivo, analgesic effects of low doses of primidone were demonstrated in mice, applying PregS- and heat-induced pain models, including inflammatory hyperalgesia. Thus, applying the approved drug at concentrations that are lower than those needed to induce anticonvulsive effects offers a shortcut for studying physiological and pathophysiological roles of TRPM3 in vivo.
PMID:28106668 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5402713 Krugel U et al; Pain 158 (5): 856-867 (2017)