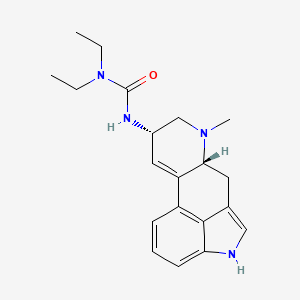

1. Arolac
2. Carbamide, Methylergol
3. Cuvalit
4. Dopergin
5. Dopergine
6. Hydrochloride, Lisuride
7. Hydrogen Maleate, Lysuride
8. Lisuride Hydrochloride
9. Lisuride Maleate
10. Lisuride Maleate (1:1)
11. Lisuride Maleate, (8beta)-isomer
12. Lisuride Mesylate
13. Lisuride Phosphate (1:1)
14. Lisuride, (8alpha)-(+-)-isomer
15. Lysenyl
16. Lysurid
17. Lysuride Hydrogen Maleate
18. Maleate, Lisuride
19. Mesylate, Lisuride
20. Methylergol Carbamide
21. Revanil
1. Lysuride
2. 18016-80-3
3. Lisuride [inn]
4. Lisurida
5. Lisuridum
6. Lisuride Maleate
7. Lisuride (inn)
8. 140387-89-9
9. Lisuride (s)(-)
10. N'-((8alpha)-9,10-didehydro-6-methylergolin-8-yl)-n,n-diethylurea
11. S-(-)-lisuride
12. Chembl157138
13. E0qn3d755o
14. Chebi:51164
15. 1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea
16. 18016-80-3 (free Base)
17. Lisuridum [inn-latin]
18. Lysuride Maleate
19. Lisurida [inn-spanish]
20. N,n-diethyl-n'-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea
21. Dopergin
22. Dl-lisuride
23. 1,1-diethyl-3-((6ar,9s)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinolin-9-yl)urea
24. 3-[(6ar,9s)-7-methyl-6,6a,8,9-tetrahydro-4h-indolo[4,3-fg]quinolin-9-yl]-1,1-diethylurea
25. 3,3-diethyl-1-[(4s,7r)-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaen-4-yl]urea
26. Lisuride [inn:ban]
27. Einecs 241-925-1
28. Unii-e0qn3d755o
29. (+)-lisuride
30. H8g
31. Lisuride, (s)
32. Prestwick_525
33. 3-(9,10-didehydro-6-methylergolin-8alpha-yl)-1,1-diethylurea
34. Lisuride [mi]
35. Prestwick0_000106
36. Prestwick1_000106
37. Prestwick2_000106
38. Prestwick3_000106
39. Urea, N'-((8alpha)-9,10-didehydro-6-methylergolin-8-yl)-n,n-diethyl-
40. Gtpl43
41. Lisuride [who-dd]
42. Lopac0_000751
43. Schembl43950
44. Bspbio_000092
45. Spbio_002031
46. Bpbio1_000102
47. Dtxsid3023217
48. Dtxsid30274075
49. Hms1568e14
50. Zinc3831001
51. Bdbm50056445
52. Ccg-204836
53. Db00589
54. Ncgc00179663-02
55. Hy-12713
56. Cs-0012290
57. D08132
58. Q424446
59. 1,1-diethyl-3-(9,10-didehydro-6-methyl-8alpha-ergolinyl)urea
60. 3-(9,10-didehydro-6-methyl-8alpha-ergolinyl)-1,1-diethylurea
61. 3-(9,10-didehydro-6-methylergolin-8.alpha.-yl)-1,1-diethylurea
62. N''-((8alpha)-9,10-didehydro-6-methylergolin-8-yl)-n,n-diethylurea
| Molecular Weight | 338.4 g/mol |
|---|---|
| Molecular Formula | C20H26N4O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 338.21066147 g/mol |
| Monoisotopic Mass | 338.21066147 g/mol |
| Topological Polar Surface Area | 51.4 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 544 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the management of Parkinson's Disease
There is evidence that lisuride lowers prolactin levels and, in low doses, prevents migraine attacks.
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CB - Prolactine inhibitors
G02CB02 - Lisuride
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CA - Ergot alkaloids
N02CA07 - Lisuride
Lisuride is an anti-Parkinson drug chemically related to the dopaminergic ergoline Parkinson's drugs. Lisuride binds to the 5-HT(1A) and 5-HT(2A/2C) receptors. It is also thought to bind to the dopamine receptor and to act as a dopamine agonist. Evidence has also emerged that Lisuride also binds to the Histamine H1 receptor.