


1. Acid, Isolithocholic
2. Acid, Lithocholic
3. Isolithocholic Acid
4. Lithocholate
1. Lithocolic Acid
2. Lithocholate
3. 3alpha-hydroxy-5beta-cholan-24-oic Acid
4. 3alpha-hydroxy-5beta-cholanic Acid
5. 3alpha-hydroxycholanic Acid
6. 3-alpha-hydroxycholanic Acid
7. 5beta-cholanic Acid-3alpha-ol
8. 3
9. A-hydroxy-5
10. A-cholanic Acid
11. Nci-c03861
12. 3-hydroxycholan-24-oic Acid
13. 3alpha-hydroxy-5beta-cholanoic Acid
14. (3alpha,5beta)-3-hydroxycholan-24-oic Acid
15. 3-alpha-hydroxy-5-beta-cholanic Acid
16. 3alpha-hydroxy-5beta-cholanate
17. 5-beta-cholanic Acid, 3-alpha-hydroxy-
18. Cholan-24-oic Acid, 3-hydroxy-, (3alpha,5beta)-
19. 5beta-cholan-24-oic Acid, 3alpha-hydroxy-
20. Nsc683770
21. (3-alpha,5-beta)-3-hydroxycholan-24-oic Acid
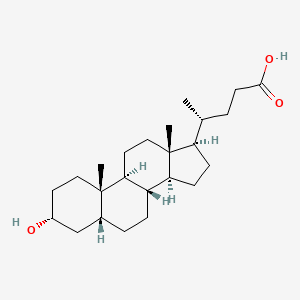
22. (4r)-4-[(3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]pentanoic Acid
23. Chembl1478
24. 17beta-(1-methyl-3-carboxypropyl)etiocholan-3alpha-ol
25. 3.alpha.-hydroxycholanic Acid
26. 17-beta-(1-methyl-3-carboxypropyl)ethiocholan-3-alpha-ol
27. Chebi:16325
28. 5-beta-cholan-24-oic Acid, 3-alpha-hydroxy-
29. Cholan-24-oic Acid, 3-hydroxy-, (3a,5b)-
30. 5qu0i8393u
31. Nsc-683770
32. Dsstox_cid_779
33. Dsstox_rid_75786
34. Dsstox_gsid_20779
35. Lca
36. Litocholic Acid
37. (3beta,5beta,14beta,17alpha)-3-hydroxycholan-24-oic Acid
38. Mfcd00003682
39. (r)-4-((3r,5r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)pentanoic Acid
40. Ccris 363
41. Lithocholicacid
42. Hsdb 4113
43. Sr-05000000450
44. Einecs 207-099-1
45. Nsc 657956
46. Brn 3217757
47. Cholan-24-oic Acid, 3-hydroxy-, (3.alpha.,5.beta.)-
48. Unii-5qu0i8393u
49. Lithocholic-acid
50. Nsc657956
51. Nsc-657956
52. Prestwick_88
53. 4oa
54. 5beta-cholan-24-oic Acid-3alpha-ol
55. Nsc 683770
56. Lithocholic Acid,(s)
57. 4q0a
58. St069335
59. Prestwick0_000796
60. Prestwick1_000796
61. Prestwick2_000796
62. Prestwick3_000796
63. Spectrum5_002021
64. Bmse000686
65. Upcmld-dp153
66. Cid_9903
67. Lithocholic Acid, >=95%
68. Bidd:pxr0054
69. Schembl28449
70. 3a-hydroxy-5b-cholanic Acid
71. Bspbio_000932
72. Gtpl611
73. 4-10-00-00785 (beilstein Handbook Reference)
74. Mls002154006
75. 3a-hydroxy-5ss-cholanic Acid
76. Lithocholic Acid [mi]
77. Spbio_002871
78. Bpbio1_001026
79. Cholan-24-oic Acid, 3-hydroxy-, (3-alpha,5-beta)-
80. 3a-hydroxy-5b-cholan-24-oate
81. Lithocholic Acid [hsdb]
82. Dtxsid6020779
83. Upcmld-dp153:001
84. 5ss--cholan-24-oic Acid-3a-ol
85. Hms1570o14
86. Hms2097o14
87. Hms2269c14
88. Hms3714o14
89. 3a-hydroxy-5b-cholan-24-oic Acid
90. Hy-b0172
91. Zinc3918156
92. Tox21_201868
93. Tox21_302791
94. 3a-hydroxy-5ss-cholan-24-oic Acid
95. 5.beta.-cholanic Acid-3.alpha.-ol
96. Bdbm50236238
97. Lmst04010003
98. S4003
99. (3a,5b)-3-hydroxy-cholan-24-oate
100. Akos016010251
101. Lithocholic Acid [ep Impurity]
102. Ccg-220796
103. Cs-2049
104. Ds-3878
105. 3.alpha.-hydroxy-5.beta.-cholanic Acid
106. Ncgc00091272-01
107. Ncgc00091272-04
108. Ncgc00091272-06
109. Ncgc00091272-07
110. Ncgc00091272-08
111. Ncgc00256451-01
112. Ncgc00259417-01
113. (3a,5b)-3-hydroxy-cholan-24-oic Acid
114. (4s)-4-((1s,2s,11s,5r,7r,10r,14r,15r)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0. 0<2,7>.0<11,15>]heptadec-14-yl)pentanoic Acid
115. 3.alpha.-hydroxy-5.beta.-cholanoic Acid
116. Nci60_028903
117. Nci60_030095
118. Smr000112168
119. 5.beta.-cholan-24-oic Acid-3.alpha.-ol
120. L0089
121. 5-.beta.-cholanic Acid, 3-.alpha.-hydroxy-
122. 3.alpha.-hydroxy-5.beta.-cholan-24-oic Acid
123. C03990
124. 5.beta.-cholan-24-oic Acid, 3.alpha.-hydroxy-
125. A872700
126. Q3323035
127. Sr-05000000450-2
128. Sr-05000000450-4
129. Sr-05000000450-5
130. Ursodeoxycholic Acid Impurity C [ep Impurity]
131. 17-.beta.-(1-methyl-3-carboxypropyl)ethiocholan-3-.alpha.-ol
132. 3alpha-hydroxy-5beta-cholan-24-oic Acid (lithocholic Acid)
133. Cholan-24-oic Acid, 3-hydroxy-, (3-.alpha., 5-.beta.)-
134. Cholan-24-oic Acid, 3-hydroxy-, (3-alpha,5-beta)- (9ci)
135. 17.beta.-(1-methyl-3-carboxypropyl)etiocholan-3.alpha.-ol
136. Lithocholic Acid, European Pharmacopoeia (ep) Reference Standard
137. Ac268b61-0548-4391-90e9-546636926870
138. Lithocholic Acid, 50 Mug/ml In Methanol, Certified Reference Material
139. (4r)-4-((3r,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)pentanoic Acid
140. 3alpha-hydroxy-5beta-cholan-24-oic Acid 3alpha-hydroxy-5beta-cholanic Acid 3alpha-hydroxycholanic Acid 3-hydroxycholanic Acid 5beta-cholan-24-oic Acid-3alpha-ol 5beta-cholan-24-oic Acid-3a-ol 5beta-cholanic Acid-3alpha-ol Beta-cholanic Acid-3-alpha-ol Hydroycholanic Acid
| Molecular Weight | 376.6 g/mol |
|---|---|
| Molecular Formula | C24H40O3 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 376.29774513 g/mol |
| Monoisotopic Mass | 376.29774513 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Detergents
Purifying or cleansing agents, usually salts of long-chain aliphatic bases or acids, that exert cleansing (oil-dissolving) and antimicrobial effects through a surface action that depends on possessing both hydrophilic and hydrophobic properties. (See all compounds classified as Detergents.)
LITHOCHOLIC ACID (24)C(14) IS CONVERTED BY RAT LIVER HOMOGENATE INTO 3ALPHA-6BETA-DIHYDROXY-5BETA-CHOLANIC ACID, 7SIGMA-HYDROXYLATION OCCURS, HYDROXYLATION CONJUGATION WITH TAURINE & FORMATION OF 3-SULFATE ESTER CAN BE DEMONSTRATED.
BACK P, SCHERNAU-POTZI L; NAUNYN-SCHMIEDEBERG'S ARCH PHARMACOL 275 (2): 135 (1972)
LABELED LITHOCHOLATE WAS INJECTED INTO GALLSTONE PATIENTS & HEALTHY VOLUNTEERS, MAJORITY OF RADIOACTIVITY IN BILE (50-60%) WAS PRESENT AS SULFATED CONJUGATES. DEGREE OF SULFATION WAS GREATER FOR GLYCINE THAN TAURINE CONJUGATES, WHICH SUGGESTED PREFERENTIAL SULFATION OF GLYCINE CONJUGATES.
PMID:955496 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1411124 ALLAN RN ET AL; GUT 17 (6): 413 (1976)
Lithocholic Acid has known human metabolites that include 6alpha-Hydroxylithocholic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560