

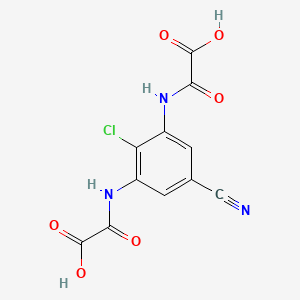

1. Diethyl N,n'-(2-chloro-5-cyano-m-phenylene)dioxamate
2. Lodoxamide Ethyl
3. U-42,718
1. 53882-12-5
2. Lodoxamidum
3. 2-[2-chloro-5-cyano-3-(oxaloamino)anilino]-2-oxoacetic Acid
4. U-42585e (free Acid)
5. Spu695od73
6. Lodoxamide (inn)
7. 2,2'-((2-chloro-5-cyano-1,3-phenylene)bis(azanediyl))bis(2-oxoacetic Acid)
8. Lodoxamide [inn]
9. Alomide (tn)
10. N,n'-(2-chloro-5-cyano-m-phenylene)dioxamate
11. Lodoxamida
12. Lodoxamide [inn:ban]
13. Lodoxamidum [inn-latin]
14. Acetic Acid, 2,2'-((2-chloro-5-cyano-1,3-phenylene)diimino)bis(2-oxo-
15. Acetic Acid, 2,2'-[(2-chloro-5-cyano-1,3-phenylene)diimino]bis[2-oxo-
16. Lodoxamida [inn-spanish]
17. Unii-spu695od73
18. N,n'-(2-chlor-5-cyan-3-phenylen)dioxamsaeure
19. Lodoxamide [mi]
20. Lodoxamide [vandf]
21. Lodoxamide [mart.]
22. Dsstox_cid_31556
23. Dsstox_rid_97441
24. Lodoxamide [who-dd]
25. Dsstox_gsid_57767
26. Schembl119881
27. Gtpl9743
28. Chembl1201266
29. Dtxsid9057767
30. Chebi:135333
31. Hms3715i21
32. Zinc2000707
33. Tox21_113669
34. Bdbm50259889
35. Ccg-221010
36. Cs-6498
37. Db06794
38. Ncgc00249893-01
39. As-76953
40. Hy-14270
41. Cas-53882-12-5
42. Ft-0627968
43. D08139
44. W18254
45. 882l125
46. A901322
47. Q6666510
48. 2-[[2-chloro-5-cyano-3-(oxaloamino)phenyl]amino]-2-oxoacetic Acid
49. 2,2'-[(2-chloro-5-cyano-1,3-phenylene)diimino]bis[2-oxoacetic Acid]
50. Acetic Acid, 2,2'-((2-chloro-5-cyano-1,3-phenylene)diimino)-, Bis 2-oxo-
| Molecular Weight | 311.63 g/mol |
|---|---|
| Molecular Formula | C11H6ClN3O6 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 310.9945126 g/mol |
| Monoisotopic Mass | 310.9945126 g/mol |
| Topological Polar Surface Area | 157 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 489 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated in the treatment of the ocular disorders referred to by the terms vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
FDA Label
Lodoxamide is a mast cell stabilizer that inhibits the in vivo Type 1 immediate hypersensitivity reaction. Lodoxamide therapy inhibits the increases in cutaneous vascular permeability that are associated with reagin or IgE and antigen-mediated reactions.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
S01GX05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX05 - Lodoxamide
Absorption
In a study of twelve healthy adult volunteers, topical administration of lodoxamide tromethamine ophthalmic solution 0.1%, one drop in each eye four times per day for ten days, did not result in any measurable lodoxamide plasma levels at a detection limit of 2.5 ng/mL.
Route of Elimination
Urinary excretion is the major route of elimination.
Elimination half-life was 8.5 hours in urine.
Although lodoxamide's precise mechanism of action is unknown, it is postulated that it prevents calcium influx into mast cells upon antigen stimulation and therefore stabilizes the membrane. By stabilizing the mast cell membrane from degranulation, lodoxamide consequently inhibits the release of intracellular histamine and other chemoattractant factors that primarily cause ocular symptoms. Lodoxamide's mechanism of action may be similar to cromolyn sodium, as both exhibit cross-tachyphylaxis.