
1. Acid Diethylamide, Lysergic
2. Diethylamide, Lysergic Acid
3. Lsd
4. Lsd 25
5. Lsd-25
6. Lysergic Acid Diethylamide
7. Lysergic Acid Diethylamide Tartrate
1. Lysergic Acid Diethylamide
2. N,n-diethyllysergamide
3. D-lysergic Acid Diethylamide
4. D-lsd
5. 50-37-3
6. Delysid
7. Lsd
8. Lysergamid
9. Lysergsaeurediaethylamid
10. N,n-diethyl-d-lysergamide
11. Lysergaure Diethylamid
12. Lsd-25
13. Lsd (alkaloid)
14. Lsd 25
15. Lysergsauerediaethylamid
16. Lysergic Acid Diethylamide-25
17. (+)-lsd
18. Lysergamide, N,n-diethyl-
19. N,n-diethyl-(+)-lysergamide
20. (+)-lysergic Acid Diethylamide
21. Hsdb 3920
22. Lysergsaeurediethylamid
23. 8na5swf92o
24. [3h]-lsd
25. Chebi:6605
26. Chembl263881
27. Cubes
28. Pearly Gates
29. Heavenly Blue
30. Royal Blue
31. Acid [street Name]
32. Dextrolysergic Acid Diethylamide
33. Cubes [street Name]
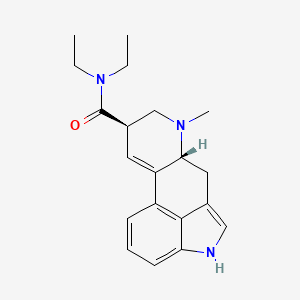
34. Ncgc00168265-01
35. Ncgc00168265-02
36. Dsstox_cid_3231
37. Royal Blue [street Name]
38. Lysergidum [inn-latin]
39. Pearly Gates [street Name]
40. (6ar,9r)-n,n-diethyl-7-methyl-6,6a,8,9-tetrahydro-4h-indolo[4,3-fg]quinoline-9-carboxamide
41. Dsstox_rid_76934
42. Dsstox_gsid_23231
43. Wedding Bells [street Name]
44. Diethylamid Kyseliny Lysergove
45. Heavenly Blue [street Name]
46. Lisergide [dcit]
47. Clearlight
48. Lysergidum
49. Ubergluben
50. Barrels
51. Bartman
52. Cupcakes
53. Greenies
54. Lisergide
55. Lisergido
56. Microdots
57. Spoonies
58. Sunshine
59. Trippers
60. Yellows
61. Beast
62. Chembl463207
63. Chief
64. Domes
65. Fifty
66. Flats
67. Owsley
68. Scapes
69. Wedges
70. (8r)-9,10-didehydro-n,n-diethyl-6-methylergoline-8-carboxamide
71. Chocolate Chips
72. Orange Mushroom
73. Orange Sunshine
74. Purple Microdot
75. Gelatin Chips
76. Mellow Yellow
77. Orange Wedges
78. Bart Simpson
79. Blotter Acid
80. Contact Lens
81. (8alpha)-n,n-diethyl-6-methyl-9,10-didehydroergoline-8-carboxamide
82. Purple Haze
83. White Light
84. Window Pane
85. Blue Cheer
86. Brown Dots
87. Cherry Top
88. Lisergido [inn-spanish]
89. Mean Green
90. Paper Acid
91. Sugar Lump
92. Blue Acid
93. Blue Mist
94. Blue Star
95. Strawberry Fields
96. The Hawk
97. Cas-50-37-3
98. California Sunshine
99. Lysergide [inn:ban:dcf]
100. (4r,7r)-n,n-diethyl-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide
101. Diethylamid Kyseliny Lysergove [czech]
102. Big F
103. Einecs 200-033-2
104. Unii-8na5swf92o
105. Brn 0094179
106. Hawk
107. Haze
108. Lids
109. Lucy In The Sky With Diamonds
110. Instant Zen
111. Dea No. 7315
112. Wedding Bells Acid
113. 7ld
114. Ergoline-8-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-, (8beta)-
115. [n-methyl-3h]lsd
116. Lysergide [inn]
117. Lysergide [mi]
118. Lsd,l-
119. Lysergide [hsdb]
120. 9,10-didehydro-n,n-diethyl-6-methyl-ergoline-8-beta-carboxamide
121. Ergoline-8beta-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-
122. Gtpl17
123. Indolo[4,3-fg]quinoline, Ergoline-8-carboxamide Deriv.
124. Lysergide [who-dd]
125. 4-25-00-00939 (beilstein Handbook Reference)
126. Schembl113755
127. Dtxsid1023231
128. Niosh/ke4200000
129. Bdbm21342
130. Lsd,(+)
131. 9,10-didehydro-n,n-diethyl-6-methylergoline-8b-carboxamide
132. Lysergic Acid Diethylamide, L-isomer
133. Tox21_112872
134. Tox21_113508
135. Bdbm50241702
136. Ergoline-8-beta-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-
137. Ergoline-8-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-, (8b)-
138. Pdsp1_001431
139. Pdsp1_001540
140. Pdsp2_001415
141. Pdsp2_001524
142. Zinc96903803
143. Lysergic Acid Diethylamide Tartrate Ci
144. Db04829
145. (+)-lysergide (incb:green List)
146. Lsd (lysergic Acid Diethylamide;lysergide)
147. Ke42000000
148. C07542
149. Q23118
150. Lsd (lysergic Acid Diethylamide) 0.025 Mg/ml In Acetonitrile
151. Lsd (lysergic Acid Diethylamide) 1.0 Mg/ml In Acetonitrile
152. (8beta)-n,n-diethyl-6-methyl-9,10-didehydroergoline-8-carboxamide
153. 9,10-didehydro-n,n-diethyl-6-methyl-ergoline-8b-carboxamide
154. Ergoline-8-beta-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-, (-)-
155. Ergoline-8b-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl- (8ci)
156. (8.beta.)-9,10-didehydro-n,n-diethyl-6-methylergoline-8-carboxamide
157. Ergoline-8-carboxamide, 9,10-didehydro-n,n-diethyl-6-methyl-, (5.beta., 8.beta.)-
158. Lsd Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
159. Lsd Solution, 25 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
160. (6ar,9r)-n,n-diethyl-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide
| Molecular Weight | 323.4 g/mol |
|---|---|
| Molecular Formula | C20H25N3O |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 323.199762429 g/mol |
| Monoisotopic Mass | 323.199762429 g/mol |
| Topological Polar Surface Area | 39.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 527 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hallucinogens; Serotonin Agonists; Serotonin Antagonists
National Library of Medicine's Medical Subject Headings. Lysergic Acid Diethylamide. Online file (MeSH, 2017). Available from, as of October 2, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lysergic Acid Diethylamide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of October 2, 2017: https://clinicaltrials.gov/
/EXPL THER/ Psychedelic drugs such as lysergic acid diethylamide (LSD) were used extensively in psychiatry in the past and their therapeutic potential is beginning to be re-examined today. Psychedelic psychotherapy typically involves a patient lying with their eyes-closed during peak drug effects, while listening to music and being supervised by trained psychotherapists. In this context, music is considered to be a key element in the therapeutic model; working in synergy with the drug to evoke therapeutically meaningful thoughts, emotions and imagery. The underlying mechanisms involved in this process have, however, never been formally investigated. Here we studied the interaction between LSD and music-listening on eyes-closed imagery by means of a placebo-controlled, functional magnetic resonance imaging (fMRI) study. Twelve healthy volunteers received intravenously administered LSD (75 ug) and, on a separate occasion, placebo, before being scanned under eyes-closed resting conditions with and without music-listening. The parahippocampal cortex (PHC) has previously been linked with (1) music-evoked emotion, (2) the action of psychedelics, and (3) mental imagery. Imaging analyses therefore focused on changes in the connectivity profile of this particular structure. Results revealed increased PHC-visual cortex (VC) functional connectivity and PHC to VC information flow in the interaction between music and LSD. This latter result correlated positively with ratings of enhanced eyes-closed visual imagery, including imagery of an autobiographical nature. These findings suggest a plausible mechanism by which LSD works in combination with music listening to enhance certain subjective experiences that may be useful in a therapeutic context.
PMID:27084302 Kaelen M et al; Eur Neuropsychopharmacol 26 (7): 1099-109 (2016)
/EXPL THER/ A recently published study showed the safety and efficacy of LSD-assisted psychotherapy in patients with anxiety associated with life-threatening diseases. Participants of this study were included in a prospective follow-up. 12 months after finishing LSD psychotherapy, 10 participants were tested for anxiety (STAI) and participated in a semi-structured interview. A Qualitative Content Analysis (QCA) was carried out on the interviews to elaborate about LSD effects and lasting psychological changes. None of the participants reported lasting adverse reactions. The significant benefits as measured with the STAI were sustained over a 12-month period. In the QCA participants consistently reported insightful, cathartic and interpersonal experiences, accompanied by a reduction in anxiety (77.8%) and a rise in quality of life (66.7%). Evaluations of subjective experiences suggest facilitated access to emotions, confrontation of previously unknown anxieties, worries, resources and intense emotional peak experiences a la Maslow as major psychological working mechanisms. The experiences created led to a restructuring of the person's emotional trust, situational understanding, habits and world view. LSD administered in a medically supervised psychotherapeutic setting can be safe and generate lasting benefits in patients with a life-threatening disease. Explanatory models for the therapeutic effects of LSD warrant further study.
PMID:25389218 Gasser P et al; J Psychopharmacol 29 (1): 57-68 (2015)
For more Therapeutic Uses (Complete) data for LSD (7 total), please visit the HSDB record page.
Serotonin Receptor Agonists
Endogenous compounds and drugs that bind to and activate SEROTONIN RECEPTORS. Many serotonin receptor agonists are used as ANTIDEPRESSANTS; ANXIOLYTICS; and in the treatment of MIGRAINE DISORDERS. (See all compounds classified as Serotonin Receptor Agonists.)
Hallucinogens
Drugs capable of inducing illusions, hallucinations, delusions, paranoid ideations, and other alterations of mood and thinking. Despite the name, the feature that distinguishes these agents from other classes of drugs is their capacity to induce states of altered perception, thought, and feeling that are not experienced otherwise. (See all compounds classified as Hallucinogens.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Absorption
Rapidly absorbed.
/LSD/ is converted metabolized in the liver via hydroxylation and glucuronidation, and excreted predominantly as a pharmacologically inactive compound.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1117
When taken orally, the effects become apparent within about 30 minutes and may continue for 8 to 12 hours or more. The duration and intensity of effects are dose-dependent.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); Drug Profiles: Lysergide (LSD). Available from, as of November 6, 2017: https://www.emcdda.europa.eu/drug-profiles
Following a dose of 160 ug to 13 subjects, plasma concentrations varied considerably up to 9 ug/L. Only about 1 % is excreted unchanged in the urine in 24 hours.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); Drug Profiles: Lysergide (LSD). Available from, as of November 6, 2017: https://www.emcdda.europa.eu/drug-profiles
The qualitative and quantitative aspects of the metabolism and elimination of (14)C-LSD in the rat, guinea pig and rhesus monkey have been investigated. Rats given an i.p. dose (1 mg/kg) excreted 73% of the (14)C in the feces, 16% in the urine and 3.4% in the expired air as (14)CO2 in 96 hr. Guinea pigs similarly dosed, excreted 40% in the feces, 28% (urine) and 18% (expired (14)CO2) in 96 hr. Rhesus monkeys (0.15 mg/kg i.m.) eliminated 39% of the (14)C in the urine and 23% in the feces in 96 hr. Extensive biliary excretion of (14)C-LSD occurred in both the rat and guinea pig. Bile duct-cannulated rats excreted 68% of an i.v. dose (1.33 mg/kg) in the bile in 5 hr and the guinea pig 52% in 6 hr...
PMID:117811 Siddik ZH et al.; Biochem Pharmacol 28 (20): 3093-101 (1979)
For more Absorption, Distribution and Excretion (Complete) data for LSD (8 total), please visit the HSDB record page.
Hepatic.
Three case reports are presented, including autopsy findings and toxicological screening results, which were tested positive for the potent hallucinogenic drug lysergic acid diethylamide (LSD). LSD and its main metabolites were quantified in brain tissue and femoral blood, and furthermore hematoma and urine when available. LSD, its main metabolite 2-oxo-3-hydroxy-LSD (oxo-HO-LSD), and iso-LSD were quantified in biological samples according to a previously published procedure involving liquid-liquid extraction and ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). ...
PMID:28803722 Mardal M et al; Forensic Sci Int 278: e14-e18 (2017)
Following a dose of 160 ug to 13 subjects ... in humans, LSD is extensively transformed in the liver by hydroxylation and glucuronide conjugation to inactive metabolites. ...A major metabolite found in urine is 2-oxylysergide.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); Drug Profiles: Lysergide (LSD). Available from, as of November 6, 2017: https://www.emcdda.europa.eu/drug-profiles
The metabolism of lysergic acid diethylamide (LSD) to 2-oxo-3-hydroxy lysergic acid diethylamide (O-H-LSD) was investigated in liver microsomes and cyropreserved hepatocytes from humans. Previous studies have demonstrated that O-H-LSD is present in human urine at concentrations 16-43 times greater than LSD, the parent compound. Additionally, these studies have determined that O-H-LSD is not generated during the specimen extraction and analytical processes or due to parent compound degradation in aqueous urine samples. However, these studies have not been conclusive in demonstrating that O-H-LSD is uniquely produced during in vivo metabolism. Phase I drug metabolism was investigated by incubating human liver microsomes and cryopreserved human hepatocytes with LSD. The reaction was quenched at various time points, and the aliquots were extracted using liquid partitioning and analyzed by liquid chromatography-mass spectrometry. O-H-LSD was positively identified in all human liver microsomal and human hepatocyte fractions incubated with LSD. In addition, O-H-LSD was not detected in any microsomal or hepatocyte fraction not treated with LSD nor in LSD specimens devoid of microsomes or hepatocytes. This study provides definitive evidence that O-H-LSD is produced as a metabolic product following incubation of human liver microsomes and hepatocytes with LSD.
PMID:11043658 Klette KL et al; J Anal Toxicol 24 (7): 550-6 (2000)
The qualitative and quantitative aspects of the metabolism and elimination of (14)C-LSD in the rat, guinea pig and rhesus monkey have been investigated. ...(14)C-LSD is almost completely metabolized by all three species and little unchanged drug is excreted. The metabolites identified were 13- and 14-hydroxy-LSD and their glucuronic acid conjugates, 2-oxo-LSD, de-ethyl LSD and a naphthostyril derivative. There occur, however, important species differences in the nature and amounts of the various metabolites. In the rat and guinea pig the major metabolites were the glucuronic acid conjugates of 13- and 14-hydroxy-LSD which were found in both urine and bile. The guinea pig excreted significant amounts of 2-oxo-LSD in urine and bile. De-ethyl LSD was a minor urinary metabolite in both species. The metabolism of LSD appeared to be more complicated in the rhesus monkey. The urine contained at least nine metabolites of which four were identified as follows: 13- and 14-hydroxy-LSD (as glucuronic acid conjugates) de-ethyl LSD and a naphthostyril derivative. Unlike the rat and guinea pig the glucuronic acid conjugates of 13- and 14-hydroxy-LSD were only present in small amounts. Of the remaining five unidentified metabolites, three were major. The biliary metabolites of (14)C-iso-LSD in the rat have been studied and been shown to be similar to those produced from (14)C-LSD, namely 13- and 14-hydroxy-iso-LSD and their glucuronic acid conjugates and 2-oxo-iso-LSD.
PMID:117811 Siddik ZH et al.; Biochem Pharmacol 28 (20): 3093-101 (1979)
For more Metabolism/Metabolites (Complete) data for LSD (7 total), please visit the HSDB record page.
3 hours
Two micrograms per kilogram of LSD-25 was administered intravenously to five normal human subjects. The concentration of drug in plasma was determined serially over the subsequent 8 hours. LSD-25 was found to be present in human plasma in relatively large quantities during the period of peak effect. The half-life of LSD-25 in human plasma was calculated to be 175 min.
PMID:14209776 Aghajanian GK, Bing OHL; Clin Pharmacol Ther 5: 611-614 (1964)
LSD has an elimination half life of about 2.5 hours.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1117
The goal of the present study was to better delineate the mechanisms of action of the prototypical hallucinogen LSD. LSD (0.03, 0.1 and 0.3 mg/kg, s.c.) produced locomotor hyperactivity, disruption of /prepulse inhibition (PPI)/ and a number of behaviors indicative of 5-HT activation such as wet-dog shakes, back muscle contractions and forepaw treading. These various behavioral effects of LSD were studied in both Sprague-Dawley and Wistar rats, although with the exception of back muscle contractions which were more prominent in Sprague-Dawley rats, no major strain differences were detected. The PPI disruption induced by LSD (0.1 mg/kg) in Sprague-Dawley rats was completely reversed by pretreatment with the selective 5-HT(2A) antagonist MDL 100907 (0.5 and 1 mg/kg, s.c.). In contrast, pretreatment with antagonists at 5-HT(2C), (SB 242084 (0.5 mg/kg, i.p.)); 5-HT(2B/2C) (SDZ SER 082 (1 mg/kg, s.c.)); 5-HT(1A), ((+)-WAY 100135 (1 and 20 mg/kg, s.c.)) and 5-HT(6) receptors, (RO 04-6790 (30 mg/kg, i.p.)), all failed to influence LSD-induced disruption of PPI. The dopamine DA(2like) receptor antagonist, haloperidol (0.1 and 0.2 mg/kg, s.c.), was without effect against an LSD-induced disruption of PPI. Finally, selective blockade of 5-HT(2A) but not 5-HT(2C) receptors completely abolished the locomotor hyperactivity induced by LSD. These findings provide empirical evidence to support the view that the hallucinogenic effects of LSD are mediated by a direct agonist effect at 5-HT(2A) receptors.
PMID:11557170 Ouagazzal A et al; Neuropsychopharmacology 25 (4): 565-75 (2001)
... By genetically expressing /5-HT(2A) receptor (2AR)/ only in cortex, /investigators/show that 2AR-regulated pathways on cortical neurons are sufficient to mediate the signaling pattern and behavioral response to hallucinogens. Hallucinogenic and nonhallucinogenic 2AR agonists both regulate signaling in the same 2AR-expressing cortical neurons. However, the signaling and behavioral responses to the hallucinogens are distinct. While lisuride and LSD both act at 2AR expressed by cortex neurons to regulate phospholipase C, LSD responses also involve pertussis toxin-sensitive heterotrimeric G(i/o) proteins and Src. ...
PMID:17270739 Gonzalez-Maeso J et al; Neuron 53 (3): 439-52 (2007)
Lysergic acid diethylamide (LSD) produces altered mood and hallucinations in humans and binds with high affinity to serotonin-2A (5-HT(2A)) receptors. Although LSD interacts with other receptors, the activation of 5-HT(2A) receptors is thought to mediate the hallucinogenic properties of LSD. The goal of this study was to identify the brain sites activated by LSD and to determine the influence of 5-HT(2A) receptors in this activation. Rats were pretreated with the 5-HT(2A) receptor antagonist MDL 100907 (0.3 mg/kg, i.p.) or vehicle 30 min prior to LSD (500 mg/kg, i.p.) administration and killed 3 hr later. Brain tissue was examined for Fos protein expression by immunohistochemistry. LSD administration produced a five- to eight-fold increase in Fos-like immunoreactivity in medial prefrontal cortex, anterior cingulate cortex, and central nucleus of amygdala. However, in dorsal striatum and nucleus accumbens no increase in Fos-like immunoreactivity was observed. Pretreatment with MDL 100907 completely blocked LSD-induced Fos-like immunoreactivity in medial prefrontal cortex and anterior cingulate cortex, but only partially blocked LSD-induced Fos-like immunoreactivity in amygdala. Double-labeled immunohistochemistry revealed that LSD did not induce Fos-like immunoreactivity in cortical cells expressing 5-HT(2A) receptors, suggesting an indirect activation of cortical neurons. These results indicate that the LSD activation of medial prefrontal cortex and anterior cingulate cortex is mediated by 5-HT(2A) receptors, whereas in amygdala 5-HT(2A) receptor activation is a component of the response. These findings support the hypothesis that the medial prefrontal cortex, anterior cingulate cortex, and perhaps the amygdala, are important regions involved in the production of hallucinations.
PMID:12220572 Gresch PJ; Neuroscience 114 (3): 707-13 (2002)
... The indoleamine hallucinogen D-lysergic acid diethylamide (LSD, which binds to 5-HT1A, 1B, 1D, 1E, 1F, 2A, 2C, 5, 6, 7, dopamine D1 and D2, and alpha1 and alpha2 adrenergic receptors), but not their non-hallucinogenic congeners, inhibited N-methyl-D-aspartate (NMDA)-induced inward current and NMDA receptor-mediated synaptic responses evoked by electrical stimulation of the forceps minor in pyramidal cells of the prefrontal cortical slices. The inhibitory effect of hallucinogens was mimicked by 5-HT in the presence of selective 5-HT1A and 5-HT3 receptor antagonists. The inhibitory action of ... LSD ... on the NMDA transmission was blocked by the 5-HT2A receptor antagonists R-(+)-alpha-(2, 3-dimethoxyphenyl)-1-[4-fluorophenylethyl]-4-piperidinethanol and ketanserin. However, at low concentrations ...LSD... only partially depressed the NMDA response, /and/ blocked the inhibitory effect of 5-HT, suggesting a partial agonist action. Whereas N-(4-aminobutyl)-5-chloro-2-naphthalenesulfonamide (W-7, a calmodulin antagonist) and N-[2-[[[3-(4'-chlorophenyl)- 2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4'-methoxy-benzenesulfonamide phosphate (KN-93, a Ca2+/CaM-KII inhibitor), but not the negative control 2-[N-4'methoxybenzenesulfonyl]amino-N-(4'-chlorophenyl)-2-propenyl-N -methylbenzylamine phosphate (KN-92), blocked the inhibitory action of LSD ..., the selective protein kinase C inhibitor chelerythrine was without any effect. /The authors/ conclude that phenethylamine and indoleamine hallucinogens may exert their hallucinogenic effect by interacting with 5-HT2A receptors via a Ca2+/CaM-KII-dependent signal transduction pathway as partial agonists and modulating the NMDA receptors-mediated sensory, perceptual, affective and cognitive processes.
PMID:10510170 Arvanov VL et al; Eur J Neurosci 11 (9): 3064-72 (1999)
For more Mechanism of Action (Complete) data for LSD (9 total), please visit the HSDB record page.