


1. 0211, Ru
2. 0211, Spi
3. Amitiza
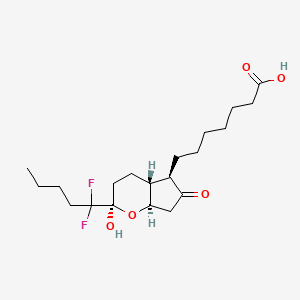
4. Prostan-1-oic Acid, 16,16-difluoro-11-hydroxy-9,15-dioxo-, (11alpha)-
5. Ru 0211
6. Ru-0211
7. Ru0211
8. Spi 0211
9. Spi-0211
10. Spi0211
1. Amitiza
2. 333963-40-9
3. 136790-76-6
4. Ru-0211
5. Spi-0211
6. (2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-heptanoic Acid
7. 7-((2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[b]pyran-5-yl)heptanoic Acid
8. 333963-40-9 (hemiketal)
9. 7-[(2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-yl]heptanoic Acid
10. Lubiprostone [usan]
11. 7-[(2r,4ar,5s,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[b]pyran-5-yl]heptanoic Acid
12. 7-[(1r,3r,6r,7r)-3-(1,1-difluoropentyl)-3-hydroxy-8-oxo-2-oxabicyclo[4.3.0]non-7-yl]heptanoic Acid
13. Amitiza (tn)
14. Bicyclic Lubiprostone
15. Lubiprostone Hemiketal
16. Lubiprostone Powder
17. Ncgc00183105-01
18. Lubiprostone (hemiketal)
19. Lubiprostone [mi]
20. Lubiprostone [inn]
21. Lubiprostone [jan]
22. Dsstox_cid_28565
23. Dsstox_rid_82837
24. Lubiprostone [vandf]
25. Dsstox_gsid_48639
26. Lubiprostone [mart.]
27. (-)-7-((2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta(b)pyran-5-yl)heptanoic Acid
28. Lubiprostone [who-dd]
29. Schembl217184
30. Gtpl4242
31. Lubiprostone (jan/usan/inn)
32. Chembl1201134
33. Dtxsid80861338
34. Lubiprostone [orange Book]
35. Amy30093
36. Ex-a1771
37. Zinc4217732
38. Tox21_112986
39. Mfcd20268389
40. Akos015896639
41. Ac-1863
42. Db01046
43. Ncgc00183105-02
44. As-39360
45. Cas-136790-76-6
46. Cs-0009583
47. Cas# 333963-40-9
48. D04790
49. 963l409
50. A850935
51. A905955
52. J-006909
53. Q6695342
54. Prostan-1-oic Acid, 11,15-epoxy-16,16-difluoro-15-hydroxy-9-oxo-, (11alpha,15r)-
55. Prostan-1-oic Acid, 16,16-difluoro-11-hydroxy-9,15-dioxo-, (11.alpha.)-
56. 7-((2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[b]pyran-5-yl)heptanoicacid
57. 7-[(2r,4ar,5r,7ar)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxo-3,4,4a,5,7,7a-hexahydrocyclopenta[e]pyran-5-yl]heptanoic Acid
| Molecular Weight | 390.5 g/mol |
|---|---|
| Molecular Formula | C20H32F2O5 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 390.22178044 g/mol |
| Monoisotopic Mass | 390.22178044 g/mol |
| Topological Polar Surface Area | 83.8 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 525 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Amitiza |
| PubMed Health | Lubiprostone (By mouth) |
| Drug Classes | Laxative |
| Drug Label | Amitiza (lubiprostone) is a chloride channel activator for oral use.The chemical name for lubiprostone is ()-7-[(2 ,4a ,5 ,7a )-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[ ]pyran-5-yl]heptanoic acid. The molecular formula of lubipro... |
| Active Ingredient | Lubiprostone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 24mcg; 8mcg |
| Market Status | Prescription |
| Company | Sucampo Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Amitiza |
| PubMed Health | Lubiprostone (By mouth) |
| Drug Classes | Laxative |
| Drug Label | Amitiza (lubiprostone) is a chloride channel activator for oral use.The chemical name for lubiprostone is ()-7-[(2 ,4a ,5 ,7a )-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[ ]pyran-5-yl]heptanoic acid. The molecular formula of lubipro... |
| Active Ingredient | Lubiprostone |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 24mcg; 8mcg |
| Market Status | Prescription |
| Company | Sucampo Pharma |
For the treatment of chronic idiopathic constipation in the adult population. Also used for the treatment of irritable bowel syndrome with constipation in women who are 18 years of age or older.
FDA Label
Treatment of constipation
Chronic idiopathic constipation is generally defined by infrequent or difficult passage of stool. The signs and symptoms associated with chronic idiopathic constipation (i.e., abdominal pain or discomfort, bloating, straining, and hard or lumpy stools) may be the result of abnormal colonic motility that can delay the transit of intestinal contents and impede the evacuation of rectal contents. One approach to the treatment of chronic idiopathic constipation is the secretion of fluid into the abdominal lumen through the activation of chloride channels in the apical membrane of the gastrointestinal epithelium. Lubiprostone is a locally acting chloride channel activator that increases intestinal chloride and fluid secretion without altering sodium and potassium concentrations in the serum.
Chloride Channel Agonists
A class of drugs that stimulate chloride ion influx through cell membrane channels. (See all compounds classified as Chloride Channel Agonists.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AX - Other drugs for constipation
A06AX03 - Lubiprostone
Absorption
Lubiprostone has low systemic availability following oral administration and concentrations of lubiprostone in plasma are below the level of quantitation (10 pg/mL).
Route of Elimination
Peak plasma concentration was shown to be around 1.14 hours, with a majority of the drug excreted in the urine within 48 hours. Lubiprostone and M3 are only detected in trace amounts in human feces.
The results of both human and animal studies indicate that lubiprostone is rapidly and extensively metabolized by 15-position reduction, α-chain β-oxidation, and ω-chain ω-oxidation. These biotransformations are not mediated by the hepatic cytochrome P450 system but rather appear to be mediated by the ubiquitously expressed carbonyl reductase. M3, a metabolite of lubiprostone in both humans and animals is formed by the reduction of the carbonyl group at the 15-hydroxy moiety that consists of both α-hydroxy and β-hydroxy epimers. M3 makes up less than 10% of the dose of radiolabeled lubiprostone.
0.9 to 1.4 hours
Lubiprostone acts by specifically activating ClC-2 chloride channels, which is a normal constituent of the apical membrane of the human intestine, in a protein kinase A action independent fashion. Activation of ClC-2 chloride channels causes an efflux of chloride ions into the lumen, which in turn leads to an efflux of sodium ions through a paracellular pathway to maintain isoelectric neutrality. As a result, water follows sodium into the lumen in order to maintain isotonic equilibrium, thereby increasing intestinal fluid secretion. By increasing intestinal fluid secretion, lubiprostone increases motility in the intestine, thereby increasing the passage of stool and alleviating symptoms associated with chronic idiopathic constipation. Activation of ClC-2 chloride channels may also stimulate the recovery of muscosal barrier function by restoring tight junction protein complexes in the intestine. Patch clamp cell studies in human cell lines have indicated that the majority of the beneficial biological activity of lubiprostone and its metabolites is observed only on the apical (luminal) portion of the gastrointestinal epithelium.

