

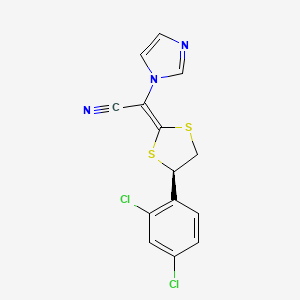

1. (2e)-((4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)(1h-imidazol-1-yl)acetonitrile
2. 4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene-1-imidazolylacetonitrile
3. Lulicon
4. Nnd 502
5. Nnd-502
6. Nnd502
1. 187164-19-8
2. Lulicon
3. Nnd-502
4. Luzu
5. Pr-2699
6. (2e)-2-[(4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile
7. Nnd 502
8. Re91an4s8g
9. Ncgc00182704-01
10. Ncgc00182704-02
11. Chebi:34825
12. (2e)-2-[(4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-yl-acetonitrile
13. (r,e)-2-(4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)-2-(1h-imidazol-1-yl)acetonitrile
14. Ncgc00182704-03
15. 2-[(2e,4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-(1h-imidazol-1-yl)acetonitrile
16. Dsstox_cid_28533
17. Dsstox_rid_82805
18. Dsstox_gsid_48607
19. (-)-(e)-((4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)(1h-imidazol-1-yl)acetonitrile
20. Luliconazole [inn]
21. Nnd502
22. Cas-187164-19-8
23. Luliconazole [usan:inn]
24. Unii-re91an4s8g
25. 4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene-1-imidazolylacetonitrile
26. Lulicon (tn)
27. Imidazole Antimycotic
28. Luzu (tn)
29. Luliconazole [mi]
30. Luliconazole [jan]
31. Luliconazole [usan]
32. Luliconazole [vandf]
33. Luliconazole [mart.]
34. Luliconazole [who-dd]
35. Schembl342362
36. Gtpl7366
37. Luliconazole (jan/usan/inn)
38. Chembl2105689
39. Dtxsid3048607
40. Luliconazole, >=98% (hplc)
41. Luliconazole [orange Book]
42. Zinc3929486
43. Tox21_112942
44. Tox21_112974
45. Mfcd00953915
46. S4258
47. Akos015897320
48. Tox21_112974_1
49. Am84645
50. Bcp9000863
51. Ccg-268075
52. Cs-0587
53. Db08933
54. Ds-3278
55. (2e)-((4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)(1h-imidazol-1-yl)acetonitrile
56. Ncgc00182704-08
57. Hy-14283
58. L0306
59. Sw219226-1
60. D01980
61. 164l198
62. A813122
63. Sr-01000945039
64. Sr-01000945039-1
65. Q15624030
66. 1h-imidazole-1-acetonitrile, Alpha-((4r)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene)-, (alphae)-
| Molecular Weight | 354.3 g/mol |
|---|---|
| Molecular Formula | C14H9Cl2N3S2 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 352.9614950 g/mol |
| Monoisotopic Mass | 352.9614950 g/mol |
| Topological Polar Surface Area | 92.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 476 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Luzu |
| PubMed Health | Luliconazole (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application.Luliconazole is (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile. Its structural formu... |
| Active Ingredient | Luliconazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Medicis |
| 2 of 2 | |
|---|---|
| Drug Name | Luzu |
| PubMed Health | Luliconazole (On the skin) |
| Drug Classes | Antifungal |
| Drug Label | LUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application.Luliconazole is (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile. Its structural formu... |
| Active Ingredient | Luliconazole |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Medicis |
Luliconazole is indicated in adults aged 18 years and older for the topical treatment of fungal infections caused by Trichophyton rubrum and Epidermophyton floccosum, specifically tinea pedis, cruris, and corporis.
FDA Label
Luliconazole kills the organisms Trichophyton rubrum and Epidermophyton floccosum, most likely by altering their fungal cell membranes.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC18 - Luliconazole
Absorption
Although luliconazole is administered topically, clinical studies have shown that after the first dose in patients with tina pedis, a maximum plasma concentration of 0.40 0.76 ng/mL (mean SD) occurred in 16.9 9.39 hours (mean SD).
Route of Elimination
The route of elimination of luliconazole has yet to be determined.
Volume of Distribution
The volume of distribution was not quantified.
Clearance
The clearance of luliconazole has yet to be determined.
The metabolism of luliconazole has yet to be determined.
The half life of luliconazole has yet to be determined.
The exact mechanism of action for luliconazole's anti-fungal activity is still not known, but luliconazole is thought to inhibit the enzyme lanosterol demethylase. Lanosterol demethylase is needed for the synthesis of ergosterol, which is a major component of the fungus cell membranes.