


1. Vx 809
2. Vx-809
3. Vx809
1. 936727-05-8
2. Vx-809
3. Vx 809
4. Vx809
5. Vrt-826809
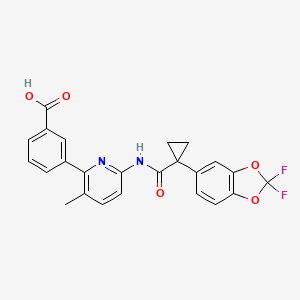
6. 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic Acid
7. Vrt 826809
8. Vx-809 (lumacaftor)
9. Egp8l81apk
10. 3-(6-{[1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanecarbonyl]-amino}-3-methyl-pyridin-2-yl)-benzoicacid
11. 3-(6-{[1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanecarbonyl]-amino}-3-methyl-pyridin-2-yl)-benzoic Acid
12. 3-[6-[[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropanecarbonyl]amino]-3-methylpyridin-2-yl]benzoic Acid
13. Lumacaftor (usan)
14. 3-(6-{[1-(2,2-difluoro-2h-1,3-benzodioxol-5-yl)cyclopropane-1-carbonyl]amino}-3-methylpyridin-2-yl)benzoic Acid
15. Lumacaftor [usan]
16. 3-(6-(1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropane-1-carboxamido)-3-methylpyridin-2-yl)benzoic Acid
17. 3-(6-[[1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanecarbonyl]-amino]-3-methyl-pyridin-2-yl)-benzoic Acid
18. 3-(6-{[1-(2,2-difluoro-benzo[1,3]dioxol-5-yl)-cyclopropanecarbonyl]-amino}-3-methyl-pyridin-2-yl)-be
19. 3-(6-{[1-(2,2-difluorobenzo[1,3]dioxol-5-yl)cyclopropanecarbonyl]-amino}-3-methyl-pyridin-2-yl)benzoic Acid
20. Benzoic Acid, 3-(6-(((1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl)carbonyl)amino)-3-methyl-2-pyridinyl)-
21. Lumacaftor [inn]
22. Lumacaftor [usan:inn]
23. Unii-egp8l81apk
24. Vx8
25. Lumacaftor [mi]
26. Lumacaftor (vx-809)
27. Lumacaftor [who-dd]
28. Lumacaftor(vx-809vx809)
29. Mls006011120
30. Schembl377028
31. Gtpl7481
32. Chembl2103870
33. Lumacaftor [orange Book]
34. Chebi:90951
35. Dtxsid30239523
36. Ex-a178
37. Hms3655e05
38. Orkambi Component Lumacaftor
39. Amy14931
40. Bcp02305
41. Bdbm50289703
42. Mfcd16659051
43. S1565
44. Zinc64033452
45. Akos015920205
46. Lumacaftor Component Of Orkambi
47. Ccg-269253
48. Cs-0479
49. Db09280
50. Pb19466
51. Ncgc00346550-01
52. Ncgc00346550-02
53. Ncgc00346550-05
54. Ac-23172
55. As-31756
56. Hy-13262
57. Smr004702901
58. Ft-0757817
59. Sw219911-1
60. A25628
61. D10134
62. J-690399
63. Q6703005
64. 3-(6-(1-(2,2-difluorobenzo(d) (1,3)dioxyl-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic Acid
65. 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) Cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic Acid
66. 3-(6-{[1-(2,2-difluoro-2h-1,3-benzodioxol-5-yl)cyclopropane-1-carbonyl]amino}-3-methylpyridin-2-yl)benzoic Acid; Vx-809
67. 3-(6-{[1-(2,2-difluorobenzo[1,3]dioxol-5-yl)cyclopropanecarbonyl]amino}-3-methyl-pyridin-2-yl)benzoic Acid
| Molecular Weight | 452.4 g/mol |
|---|---|
| Molecular Formula | C24H18F2N2O5 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 452.11837800 g/mol |
| Monoisotopic Mass | 452.11837800 g/mol |
| Topological Polar Surface Area | 97.8 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 776 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
When given in combination with [DB08820] as the fixed dose combination product Orkambi, lumacaftor is indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation in the CFTR gene.
FDA Label
Treatment of cystic fibrosis
Results from clinical trials indicated that treatment with Orkambi (lumacaftor/ [DB08820]) results in improved lung function, reduced chance of experiencing a pulmonary exacerbation, reduced sweat chloride, increased weight gain, and improvements in CF symptoms and quality of life. Orkambi was not found to increase the QTc interval to any clinically relevant extent.
R07AX30
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Absorption
Following administration of Orkambi (lumacaftor/[DB08820]) with fat containing foods, peak plasma concentrations were reached at 4 hours (Tmax). It's recommended that Orkambi should be taken with fat-containing foods as they increase absorption of lumacaftor by approximately 2-fold, and[DB08820 by 3-fold.
Route of Elimination
Lumacaftor is primarily excreted unchanged in the feces (51%). A minimal amount of the parent compound and its metabolites are excreted in the urine.
Volume of Distribution
Following oral administration of 200 mg of lumacaftor every 24 hours to cystic fibrosis patients in a fed state for 28 days, the mean (+/-SD) for apparent volumes of distribution was 86.0 (69.8) L.
Clearance
The typical apparent clearance, CL/F (CV), of lumacaftor was estimated to be 2.38 L/hr.
Lumacaftor is mostly excreted unchanged in the feces and is not extensively metabolized. When metabolism does occur, oxidation and glucuronidation are the main processes involved.
The half-life of lumacaftor is approximately 26 hours.
Lumacaftor improves CF symptoms and underlying disease pathology by aiding the conformational stability of F508del-mutated CFTR, resulting in increased processing and trafficking of mature protein to the cell surface. More specifically, lumacaftor acts as a protein-folding chaperone, preventing misfolding of CFTR ion channels and consequent destruction during processing in the endoplasmic reticulum.