
1. 13496, Sm
2. Hcl, Lurasidone
3. Hydrochloride, Lurasidone
4. Latuda
5. Lurasidone
6. Lurasidone Hcl
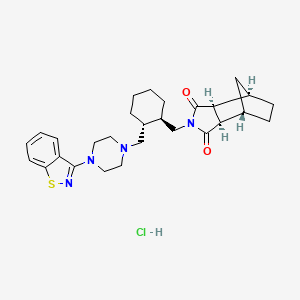
7. N-(2-(4-(1,2-benzisothiazol-3-yl)-1-piperazinylmethyl)-1-cyclohexylmethyl)-2,3-bicyclo(2.2.1)heptanedicarboximide
8. Sm 13,496
9. Sm 13496
10. Sm-13,496
11. Sm-13496
12. Sm13,496
13. Sm13496
1. Lurasidone Hcl
2. 367514-88-3
3. Latuda
4. Sm-13496
5. Lurasidone Hydrochloride [usan]
6. Sm 13496
7. Chebi:70732
8. O0p4i5851i
9. Lurasidonhydrochloride
10. (1s,2r,6s,7r)-4-[[(1r,2r)-2-[[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]methyl]cyclohexyl]methyl]-4-azatricyclo[5.2.1.02,6]decane-3,5-dione;hydrochloride
11. (3ar,4s,7r,7as)-2-(((1r,2r)-2-((4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)methyl)cyclohexyl)methyl)hexahydro-1h-4,7-methanoisoindole-1,3(2h)-dione Hydrochloride
12. (1r,2s,6r,7s)-4-{[(1r,2r)-2-{[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]methyl}cyclohexyl]methyl}-4-azatricyclo[5.2.1.0^{2,6}]decane-3,5-dione Hydrochloride
13. 4,7-methano-1h-isoindole-1,3(2h)-dione, 2-(((1r,2r)-2-((4-(1,2-benzisothiazol-3-yl)-1-piperazinyl)methyl)cyclohexyl)methyl)hexahydro-, Monohydrochloride, (3ar,4s,7r,7as)-
14. Unii-o0p4i5851i
15. Latuda (tn)
16. Lurasidone Monohydrochloride
17. Lurasidone-d8 Hydrochloride
18. Sm-13496 (hydrochloride)
19. Schembl1534132
20. Chembl1615372
21. Dtxsid401027714
22. Lurasidone Hydrochloride (jan/usan)
23. Lurasidone Hydrochloride [mi]
24. S3044
25. Smp-13496
26. Lurasidone Hydrochloride [jan]
27. Akos022185856
28. Ex-3125
29. Mk-3756
30. Lurasidone Hydrochloride [mart.]
31. Lurasidone Hydrochloride [vandf]
32. Lurasidone Hydrochloride [who-dd]
33. As-35074
34. Lurasidone Hydrochloride, >=98% (hplc)
35. Lurasidone Hydrochloride [orange Book]
36. D04820
37. Q27882070
38. (3ar,4s,7r,7as)-2-((1r,2r)-2-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl)cyclohexylmethyl)hexahydro-4,7-methano-2h-isoindole-1,3-dione, Hydrochloride
39. (3ar,4s,7r,7as)-2-{(1r,2r)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl]cyclohexylmethyl}hexahydro-4,7-methano-2h-isoindole-1,3-dione Hydrochloride
40. (3ar,4s,7r,7as)-2-{[(1r,2r)-2-{[4-(1,2-benzothiazol-3-yl)piperazin-1-yl]methyl}cyclohexyl]methyl}hexahydro-1h-4,7-methanoisoindole-1,3(2h)-dione Hydrochloride
41. 4-(1,2-benzothiazol-3-yl)-1-{[(1r,2r)-2-{[(3ar,4s,7r,7as)-1,3-dioxooctahydro-2h-4,7-methanoisoindol-2-yl]methyl}cyclohexyl]methyl}piperazin-1-ium Chloride
42. Lurasidone Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 529.1 g/mol |
|---|---|
| Molecular Formula | C28H37ClN4O2S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 528.2325753 g/mol |
| Monoisotopic Mass | 528.2325753 g/mol |
| Topological Polar Surface Area | 85 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 804 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Latuda |
| PubMed Health | Lurasidone (By mouth) |
| Drug Classes | Antipsychotic, Central Nervous System Agent |
| Drug Label | LATUDA is a psychotropic agent belonging to the chemical class of benzoisothiazol derivatives. Its chemical name is (3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl] cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3... |
| Active Ingredient | Lurasidone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 120mg; 60mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Sunovion Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Latuda |
| PubMed Health | Lurasidone (By mouth) |
| Drug Classes | Antipsychotic, Central Nervous System Agent |
| Drug Label | LATUDA is a psychotropic agent belonging to the chemical class of benzoisothiazol derivatives. Its chemical name is (3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl] cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3... |
| Active Ingredient | Lurasidone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 120mg; 60mg; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Sunovion Pharms |
Treatment of schizophrenia in adults aged 18 years and over.
Treatment of schizophrenia
Adrenergic alpha-2 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine D2 Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of DOPAMINE D2 RECEPTORS. (See all compounds classified as Dopamine D2 Receptor Antagonists.)
Serotonin 5-HT2 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT2 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT2 RECEPTOR AGONISTS. Included under this heading are antagonists for one or more specific 5-HT2 receptor subtypes. (See all compounds classified as Serotonin 5-HT2 Receptor Antagonists.)
N05AE05