


1. Beta,epsilon-carotene-3, 3'-diol, (3r,3'r,6's)-
2. Beta,epsilon-carotene-3,3'-diol, (3s,3'r,6's)-
3. Gamma Lutein
4. Lutein F
5. Lutein G
6. Lutein, Gamma
1. Xanthophyll
2. 127-40-2
3. Bo-xan
4. Vegetable Lutein
5. Vegetable Luteol
6. All-trans-lutein
7. Xantofyl
8. Lutein Ester
9. Lutein A
10. All-trans-(+)-xanthophyll
11. Floraglo
12. Floraglo Lutein
13. Trans-lutein
14. Lutein, All-trans-
15. All-trans-xanthophyll
16. Oro Glo 7
17. E 161b
18. Beta,epsilon-carotene-3,3'-diol, (3r,3'r,6'r)-
19. Xanthophyll, All-trans-(+)-
20. (3r,3'r,6'r)-lutein
21. Beta,epsilon-carotene-3,3'-diol
22. Os 24
23. Lutein From Tagetes Erecta
24. Ins No.161b(i)
25. X72a60c9mt
26. E 161
27. Chebi:28838
28. Ins-161b(i)
29. E-161b
30. E-161b(i)
31. Nsc-59193
32. .beta.,.epsilon.-carotene-3,3'-diol
33. .beta.,.epsilon.-carotene-3,3'-diol, (3r,3'r,6'r)-
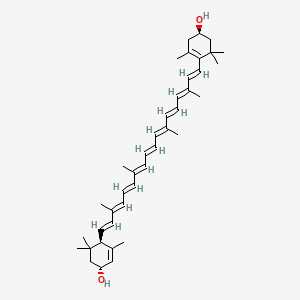
34. (1r)-4-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-[(1r,4r)-4-hydroxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethylcyclohex-3-en-1-ol
35. (1r,4r)-4-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-((r)-4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl)-3,5,5-trimethylcyclohex-2-enol
36. (3r,3'r,6'r)-beta,epsilon-carotene-3,3'-diol
37. 180580-60-3
38. 9-cis-lutein
39. Lutamax
40. 13-cis-lutein
41. Unii-x72a60c9mt
42. Leutein
43. Ncgc00167965-01
44. 15-cis-lutein
45. Einecs 204-840-0
46. 13'-cis-lutein
47. Nsc 59193
48. Lutein (xanthophyll)
49. (9'z)-lutein
50. Lutein - 5%
51. Leutein [vandf]
52. Xanthophyll (~80%)
53. Lutein [vandf]
54. E-carotene-3,3'-diol
55. Lutein - 10%
56. Lutein - 20%
57. Lutein [dsc]
58. Lutein [fcc]
59. Xanthophyll [mi]
60. Lutein [usp-rs]
61. Lutein [mart.]
62. Xanthophyll, From Marigold
63. Xantofyl [who-dd]
64. Lutein, Analytical Standard
65. Dsstox_cid_26749
66. Dsstox_rid_81874
67. Dsstox_gsid_46749
68. Schembl19342
69. 3,3'-dihydroxy-alpha-carotene
70. Chembl173929
71. Dtxsid8046749
72. (invertedexclamationmarka)-lutein
73. Bcbcmap01_000190
74. Hms3886i13
75. 29414-89-9
76. Hy-n6947
77. Zinc8221225
78. (3r,3'r)-dihydroxy-alpha-carotene
79. Tox21_112594
80. ( Inverted Exclamation Marka)-lutein
81. Bbl101804
82. Lmpr01070274
83. Mfcd00017353
84. S5103
85. Stl555601
86. Akos008901394
87. Ccg-270087
88. Db00137
89. Smp1_000317
90. As-63011
91. Cas-127-40-2
92. Xl176941
93. Xl176947
94. Xl176948
95. Cs-0015250
96. C08601
97. Q63409232
98. Lutein Solution, 1 Mg/l In Ethanol, Analytical Standard
99. 4',5'-didehydro-6'-hydro-.beta.-carotene-3,3'-diol #
100. Ab972dac-e626-49f1-898d-598af7729fd0
101. (3r,3'r,6r)-4,5-didehydro-5,6-dihydro-beta,beta-carotene-3,3'-diol
102. Lutein, Pharmaceutical Secondary Standard; Certified Reference Material
103. (3r,3'r,6r)-4,5-didehydro-5,6-dihydro-.beta.,.beta.-carotene-3,3'-diol
104. (3r,3'r,6r)-4,5-didehydro-5,6-dihydro-.beta.,.beta.-carotin-3,3'-diol
105. .beta.,.beta.-carotene-3,3'-diol, 4,5-didehydro-5,6-dihydro-, (3r,3'r,6r)-
106. (1r)-4-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-[(1r,4r)-4-hydroxy-2,6,6-trimethyl-cyclohex-2-en-1-yl]-3,7,12,16-tetramethyl-octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethyl-cyclohex-3-en-1-ol
107. (1r,4r)-4-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-18-((4r)-4-hydroxy-2,6,6-trimethyl-1-cyclohexen-1-yl)-3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaen-1-yl)-3,5,5-trimethyl-2-cyclohexen-1-ol
| Molecular Weight | 568.9 g/mol |
|---|---|
| Molecular Formula | C40H56O2 |
| XLogP3 | 11 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 10 |
| Exact Mass | 568.42803102 g/mol |
| Monoisotopic Mass | 568.42803102 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 9 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Xanthophylls are taken for nutritional supplementation, and also for treating dietary shortage or imbalance.
Lutein was found to be present in a concentrated area of the macula, a small area of the retina responsible for central vision. The hypothesis for the natural concentration is that lutein helps protect from oxidative stress and high-energy light. Several studies show that an increase in macula pigmentation decreases the risk for eye diseases such as Age-related Macular Degeneration (AMD).
Xanthophylls have antioxidant activity and react with active oxygen species, producing biologically active degradation products. They also can inhibit peroxidation of membrane phospholipids and reduce lipofuscin formation, both of which contribute to their antioxidant properties. Lutein is naturally present in the macula of the human retina. It filters out potentially phototoxic blue light and near-ultraviolet radiation from the macula. The protective effect is due in part, to the reactive oxygen species quenching ability of these carotenoids. Lutein is more stable to decomposition by pro-oxidants than are other carotenoids such as beta-carotene and lycopene. Lutein is abundant in the region surrounding the fovea, and lutein is the predominant pigment at the outermost periphery of the macula. Zeaxanthin, which is fully conjugated (lutein is not), may offer somewhat better protection than lutein against phototoxic damage caused by blue and near-ultraviolet light radiation. Lutein is one of only two carotenoids that have been identified in the human lens, may be protective against age-related increases in lens density and cataract formation. Again, the possible protection afforded by lutein may be accounted for, in part, by its reactive oxygen species scavenging abilities. Carotenoids also provide protection from cancer. One of the mechanisms of this is by increasing the expression of the protein connexin-43, thereby stimulating gap junctional communication and preventing unrestrained cell proliferation.
