


1. 177lu-617-prostate-specific Membrane Antigen Ligand
2. 177lu-psma-617
1. 177lu-psma-617
2. G6uf363ecx
3. Vipivotide Tetraxetan Lu-177
4. 177lu-labeled Psma-617
5. Lutetium Lu-177 Vipivotide Tetraxetan
6. Vipivotide Tetraxetan Lutetium Lu-177
7. Lutetium (177lu) Vipivotide Tetraxetan
8. Lutetium (177lu) Vipivotide Tetraxetan [inn]
9. Lutetium Lu 177 Vipivotide Tetraxetan [usan]
10. Pluvicto
11. Unii-g6uf363ecx
12. Who 11429
13. Lutetium (177lu) Vipivotide Tetraxetan [who-dd]
14. 1703749-62-5
15. 1983157-55-6
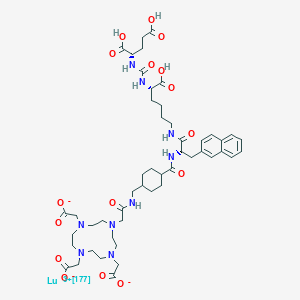
16. Lutetate(3-)-177lu, (n-((((1s)-1-carboxy-5-(((2s)-3-(2-naphthalenyl)-1-oxo-2-(((trans-4-(((2-(4,7,10-tris((carboxy-.kappa.o)methyl)-1,4,7,10-tetraazacyclododec-1-yl-.kappa.n1,.kappa.n4,.kappa.n7,.kappa.n10)acetyl-.kappa.o)amino)methyl)cyclohexyl)carbonyl)amino)propyl)amino)pentyl)amino)carbonyl)-l-glutamato(6-))-, Hydrogen (1:3)
17. Lutetate(3-)-177lu, (n2-((((1s)-1,3-dicarboxypropyl)amino)carbonyl)-n6-(3-(2-naphthalenyl)-n-((trans-4-(((2-(4,7,10-tris((carboxy-.kappa.o)methyl)-1,4,7,10-tetraazacyclododec-1-yl-.kappa.n1,.kappa.n4,.kappa.n7,.kappa.n10)acetyl-.kappa.o)amino)methyl)cyclohexyl)carbonyl)-l-alanyl)-l-lysinato(6-))-, Hydrogen (1:3
| Molecular Weight | 1216.1 g/mol |
|---|---|
| Molecular Formula | C49H68LuN9O16 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 24 |
| Exact Mass | 1215.42217 g/mol |
| Monoisotopic Mass | 1215.42217 g/mol |
| Topological Polar Surface Area | 374 Ų |
| Heavy Atom Count | 75 |
| Formal Charge | 0 |
| Complexity | 1860 |
| Isotope Atom Count | 1 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of PSMA-expressing prostate cancer
Radiopharmaceuticals
Compounds that are used in medicine as sources of radiation for radiotherapy and for diagnostic purposes. They have numerous uses in research and industry. (Martindale, The Extra Pharmacopoeia, 30th ed, p1161) (See all compounds classified as Radiopharmaceuticals.)