


1. Cg806
2. 1616428-23-9
3. Luxeptinib [usan]
4. Cg026806
5. Cg'806
6. Tq6pbx1ju0
7. Cg-026806
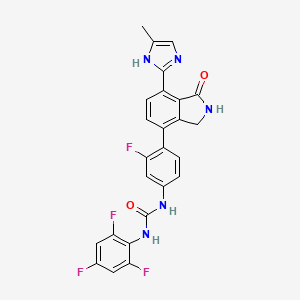
8. 1-[3-fluoro-4-[7-(5-methyl-1h-imidazol-2-yl)-1-oxo-2,3-dihydroisoindol-4-yl]phenyl]-3-(2,4,6-trifluorophenyl)urea
9. Urea, N-(4-(2,3-dihydro-7-(5-methyl-1h-imidazol-2-yl)-1-oxo-1h-isoindol-4-yl)-3-fluorophenyl)-n'-(2,4,6-trifluorophenyl)-
10. Urea, N-[4-[2,3-dihydro-7-(5-methyl-1h-imidazol-2-yl)-1-oxo-1h-isoindol-4-yl]-3-fluorophenyl]-n'-(2,4,6-trifluorophenyl)-
11. Luxeptinib [inn]
12. Unii-tq6pbx1ju0
13. Luxeptinib [who-dd]
14. Chembl4594420
15. Schembl17219212
16. Us9758508, Compound 7
17. Gtpl11671
18. Bdbm340031
19. Glxc-25679
20. Example 7 [us9758508b2]
21. Nsc791692
22. Nsc834160
23. Who 11799
24. Nsc-791692
25. Nsc-834160
26. Hy-139535
27. Cs-0204021
28. D96116
29. 1-{3-fluoro-4-[7-(5-methyl-1h-imidazol-2- Yl)-1-oxo-2,3-dihydro-1h-isoindol-4-yl]- Phenyl}-3-(2,4,6-trifluoro-phenyl)-urea
30. 3-{3-fluoro-4-[7-(4-methyl-1h-imidazol-2-yl)-1-oxo-2,3-dihydro-1h-isoindol-4-yl]phenyl-1-(2,4,6-trifluorophenyl)urea
31. 32,72,74,76-tetrafluoro-14-methyl-21,23-dihydro-11h-4,6-diaza-2(4,7)-isoindola- 1(2)-imidazola-3(1,4),7(1)-dibenzenaheptaphane-2,35-dione
| Molecular Weight | 495.4 g/mol |
|---|---|
| Molecular Formula | C25H17F4N5O2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 495.13183745 g/mol |
| Monoisotopic Mass | 495.13183745 g/mol |
| Topological Polar Surface Area | 98.9 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 811 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
