

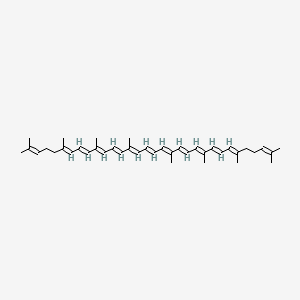
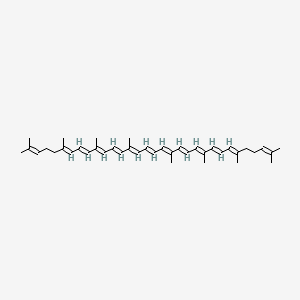
1. All Trans Lycopene
2. All-trans-lycopene
3. Lyc O Mato
4. Lyc-o-mato
5. Lycomato
6. Lycopene, (13-cis)-isomer
7. Lycopene, (7-cis,7'-cis,9-cis,9'-cis)-isomer -
8. Lycopene, (cis)-isomer
9. Pro Lycopene
10. Pro-lycopene
11. Prolycopene
1. 502-65-8
2. All-trans-lycopene
3. Psi,psi-carotene
4. Trans-lycopene
5. Lycopene 7
6. Lycored
7. Redivivo
8. Mexoryl Saq
9. Tomat-o-red
10. (6e,8e,10e,12e,14e,16e,18e,20e,22e,24e,26e)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene
11. (all-trans)-lycopene
12. Tomato Lycopene
13. Lycopene, All-trans-
14. Ateronon
15. Aec Lycopene
16. Ci 75125
17. Blakeslea Trispora
18. (all-e)-lycopene
19. Nsc 407322
20. C.i. 75125
21. Sb0n2n0wv6
22. Ins-160d(iii)
23. Ins No.160d(iii)
24. Lycopene From Blakeslea Trispora
25. Fema No. 4110
26. Chebi:15948
27. E-160d(iii)
28. Nsc-407322
29. Ncgc00166291-01
30. .psi.,.psi.-carotene
31. (all-e)-2,6,10,14,19,23,27,31-octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene
32. Lycopene (van)
33. Cis-lycopene
34. Lyc-o-mato
35. Mfcd00017350
36. Lyc
37. Ccris 7925
38. Einecs 207-949-1
39. Unii-sb0n2n0wv6
40. Lyocpenepowder
41. Nsc407322
42. Psi-psi-carotene
43. Y,y-carotene
44. Lyco Vit
45. Lycopene Preparation
46. Lycopene All-trans-
47. Lycopene [inci]
48. Lycopene [mi]
49. Lycopene [vandf]
50. Lycopene [mart.]
51. Lycopene [who-dd]
52. Dsstox_cid_26593
53. Dsstox_rid_81750
54. Dsstox_gsid_46593
55. Bspbio_003389
56. Lycopene, Analytical Standard
57. E160d
58. Tomato Lycopene [fhfi]
59. Chembl501174
60. Dtxsid2046593
61. Lycopene, >=90%, From Tomato
62. Ci 75125 [inci]
63. Act03296
64. Hy-n0287
65. Lycopene Extract From Tomato
66. Zinc8214943
67. Tox21_112395
68. Lmpr01070257
69. S3943
70. Lycopene Preparation [usp-rs]
71. Akos015961276
72. Cs-6378
73. Db11231
74. Fd10111
75. Ncgc00166291-02
76. Ncgc00166291-03
77. Ncgc00166291-04
78. Ac-13571
79. Ac-33932
80. Cas-502-65-8
81. Ls-15428
82. Lycopene, >=98% (hplc), From Tomato
83. Lycopene Extract From Tomato [fcc]
84. L0257
85. N2800
86. Lycopene From Blakeslea Trispora [fcc]
87. C05432
88. 502l658
89. Q208130
90. Q-100561
91. Lycopene, United States Pharmacopeia (usp) Reference Standard
92. Lycopene, Pharmaceutical Secondary Standard; Certified Reference Material
93. Lycopene, Redivivo(tm), 10% Fs, ~10% In Corn Oil, >=95.0% (sum Of Isomers)
94. (6e,8e,10e,12e,14e,16e,18e,20e,22e,24e,26e)-2,6,10,14,19,23,27,31-octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene
95. (all-e)-lycopene (constituent Of Lycopene And Tomato Extract Containing Lycopene) [dsc]
96. 2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene, 2,6,10,14,19,23,27,31-octamethyl-, (6e,8e,10e,12e,14e,16e,18e,20e,22e,24e,26e)-
97. 2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene, 2,6,10,14,19,23,27,31-octamethyl-, (all-e)-
98. Lyc, Lycopene, Lycopene 7, Lycopene All-trans-, Lycopene, All-trans-, Lycopene, All-trans- (8ci), Lycopene (van), Natural Yellow 27, Ncgc00166291-01, Ncgc00166291-02, Nsc407322, Nsc 407322
| Molecular Weight | 536.9 g/mol |
|---|---|
| Molecular Formula | C40H56 |
| XLogP3 | 15.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 16 |
| Exact Mass | 536.438201786 g/mol |
| Monoisotopic Mass | 536.438201786 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 11 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Radiation-Protective Agents
Drugs used to protect against ionizing radiation. They are usually of interest for use in radiation therapy but have been considered for other purposes, e.g. military. (See all compounds classified as Radiation-Protective Agents.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Anticarcinogenic Agents
Agents that reduce the frequency or rate of spontaneous or induced tumors independently of the mechanism involved. (See all compounds classified as Anticarcinogenic Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)

