


1. Aezs-130
2. Aib-dtrp-dgtrp-cho
3. Aib-trp-gtrp-cho
4. Aminoisobutyryl-tryptophyl-tryptophanamine-formyl
5. Ard-07
6. Ep 1572
7. Ep-1572
8. Ep1572
9. Jmv 1843
10. Jmv-1843
11. Jmv1843
12. Macimorelin Acetate
13. Macrilen
1. Ard-07
2. Aezs-130
3. 381231-18-1
4. Ep-1572
5. Jmv-1843
6. Ard 07
7. Jmv 1843
8. Ep 1572
9. Ep-01572
10. Chembl278623
11. 381231-18-1 (free Base)
12. Ep1572
13. Macimorelin (usan)
14. 8680b21w73
15. Macimorelin [usan]
16. D-87575
17. 2-amino-n-((r)-1-(((r)-1-formamido-2-(1h-indol-3-yl)ethyl)amino)-3-(1h-indol-3-yl)-1-oxopropan-2-yl)-2-methylpropanamide
18. 2-amino-n-[(2r)-1-[[(1r)-1-formamido-2-(1h-indol-3-yl)ethyl]amino]-3-(1h-indol-3-yl)-1-oxopropan-2-yl]-2-methylpropanamide
19. Macimorelin [inn]
20. Macimorelin [usan:inn]
21. Unii-8680b21w73
22. Aib-dtrp-dgtrp-cho
23. Macimorelin [mi]
24. Aezs130
25. Macimorelin [who-dd]
26. Gtpl9745
27. Schembl1984708
28. Jmv1843
29. Umv1843
30. Dtxsid601045766
31. Glxc-25240
32. Bdbm50125886
33. Db13074
34. Ep01572
35. Hy-14820
36. Cs-0003577
37. D10563
38. Aminoisobutyryl-tryptophyl-tryptophanamine-formyl
39. Q15624037
40. 2-amino-n-{(r)-1-[(r)-1-formylamino-2-(1h-indol-3-yl)-ethylcarbamoyl]-2-1h-indol-3-yl-ethyl}-2-methyl-propionamide
41. N(sup 2)-(2-amino-2-methylpropanoyl-n1-((1r)-1-formamido-2-(1h-indol-3-yl)ethyl)-d-tryptophanamide
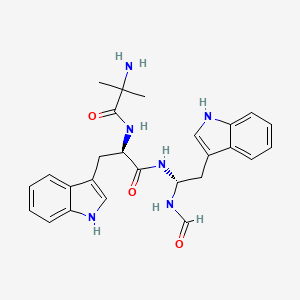
| Molecular Weight | 474.6 g/mol |
|---|---|
| Molecular Formula | C26H30N6O3 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 474.23793884 g/mol |
| Monoisotopic Mass | 474.23793884 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 761 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the diagnosis of adult growth hormone deficiency (AGHD).
FDA Label
This medicinal product is for diagnostic use only. GHRYVELIN is indicated for the diagnosis of growth hormone deficiency (GHD) in adults.
Diagnosis of growth hormone deficiency
Maximum GH levels from stimulation are observed between 30 to 90 minutes after administration of macimorelin. Increase in the QTcF interval may be observed from macimorelin administration.
V04CD06
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CD - Tests for pituitary function
V04CD06 - Macimorelin
Absorption
Macimorelin is a novel, synthetic ghrelin agonist, which is readily absorbed from the gastrointestinal tract. The maximum plasma concentration (Cmax) was observed between 0.5 and 1.5 hours following oral administration of 0.5mg/kg macimorelin to patients with AGHD under fasting for at least 8 hours. Higher doses of drug demonstrate a dose-proportional increase in plasma concentrations. A liquid meal decreased the macimorelin Cmax and AUC by 55% and 49%, respectively.
Volume of Distribution
Following a single oral dose of 0.5 mg/kg macimorelin, the mean volume of distribution of the central compartment is 5,733.4 565.7L.
Clearance
Following a single oral dose of 0.5 mg/kg macimorelin, the mean clearance over the fraction absorbed (Cl/F) was 37,411.0 4,554.6 mL/min.
Macimorelin predominantly undergoes CYP3A4-mediated metabolism according to an *in vitro* human liver microsomes study.
The mean terminal half-life (T1/2) is 4.1 hours following administration of a single oral dose of 0.5 mg macimorelin/kg body weight in healthy subjects.
Ghrelin is an endogenous ligand for the GH secretagogue receptor that is also called the ghrelin receptor (GHS-R1a). Upon activation of the receptor, ghrelin serves to increase growth hormone (GH) secretion. Macimorelin mimics the actions of ghrelin by stimulating GH release. As a synthetic agonist, it activates growth hormone secretagogue receptors present in the pituitary and hypothalamus.
